Programmed inhibition of an innate immune receptor via de novo designed transmembrane proteins
Programmed inhibition of an innate immune receptor via de novo designed transmembrane proteins
Maillie, C. A.; Zhang, M.; Goiset, N.; Goldenfeld, G.; Ward, A.; mravic, m.
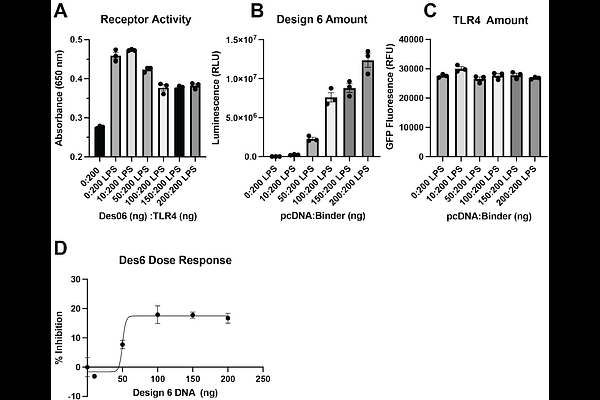
AbstractToll-like receptors (TLRs) are single-pass membrane proteins central to innate immunity, yet the role of homodimeric transmembrane (TM) domain interactions in cross-membrane signaling remains poorly understood and underexploited for therapeutic intervention. Here, we conduct a de novo computational design campaign to generate synthetic peptides that selectively target the TM domain of Toll-like receptor 4 (TLR4), a key mediator of inflammatory signaling and a clinically relevant drug target. Using in silico modeling and cell-based screening, we identified two peptides that disrupt membrane-embedded interactions essential for receptor activation. Our lead candidate binds sequence-specifically to the native TM-juxtamembrane region, acting as a largely target-selective and preliminarily dose-dependent inhibitor that impairs TLR4 signaling without affecting receptor expression. This work establishes a novel inhibitory mechanism based on structural perturbation of the TM interface and provides a generalizable framework for designing TM-targeting molecules in the absence of structural or evolutionary templates. These findings underscore the functional importance of membrane-spanning interactions and open a new therapeutic avenue for modulating TLR4 activity in the context of infection, autoimmunity, and inflammation.