AML patient blasts exhibit polarization defect upon interaction with bone marrow stromal cells.
AML patient blasts exhibit polarization defect upon interaction with bone marrow stromal cells.
Saadallah, K.; Vianay, B.; Bonnemay, L.; Pasquer, H.; Kelly, L.; Culeux, C.; Marie, R.; Fodil, S.; Chaintreuil, P.; Kerreneur, E.; Jacquel, A.; Raffoux, E.; Nizard, R.; Blanchoin, L.; Benajiba, L.; Thery, M.
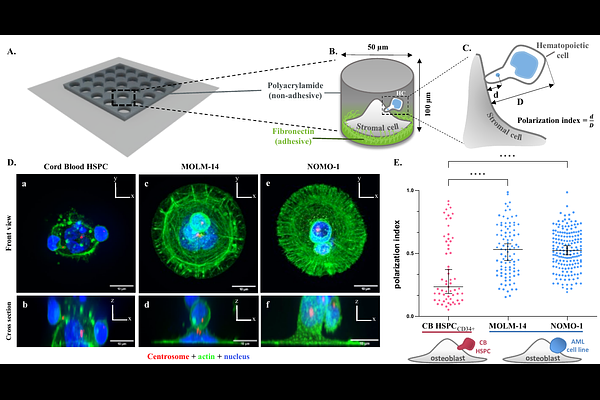
AbstractHematopoietic stem and progenitor cells (HSPCs) establish specific interactions with bone marrow stromal cells, leading to their polarization. Given the role of cell polarity in protection against tumorigenesis and the importance of the niche in hematological disorders such as acute myeloid leukemias (AMLs), we investigated the polarization capacities of leukemic blasts from patients. Using engineered micro-niches and centrosome position with respect to the contact site with stromal cells as a proxy for cell polarization, we showed that AML cell lines and primary cells from AML patient blasts were unable to polarize in contact with healthy stromal cells. In return, exposure to AML patient-derived stromal cells compromised the polarization of healthy adult HSPCs and AML blasts from patients. Using live cell imaging in engineered bone-marrow-on-a-chip, we further revealed that stromal cells from a leukemic niche increased the migration speed and distance of healthy HSPCs and AML blast as compared to their behavior in contact with healthy stromal cells. The results collectively demonstrated the respective influences of intrinsic AML blast transformation and extrinsic contact with AML stromal cells on the defective polarization of AML blast. They suggested that leukemic progression is associated with cell polarization defects and proposed new methodological approaches to investigate this relationship in AML progression.