Mechanism and Structure-Guided Optimization of SLC1A1/EAAT3-Selective Inhibitors in Kidney Cancer
Mechanism and Structure-Guided Optimization of SLC1A1/EAAT3-Selective Inhibitors in Kidney Cancer
Koochaki, P.; Qiu, B.; Coker, J. A.; Earsley, A.; Wang, N. S.; Romigh, T.; Goins, C. M.; Stauffer, S. R.; Boudker, O.; Chakraborty, A. A.
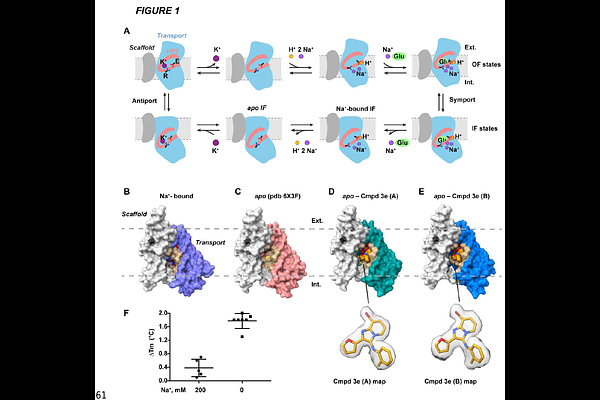
AbstractRenal Cell Carcinomas (RCCs) depend metabolically on the trimeric sodium-coupled aspartate and glutamate transporter, SLC1A1/EAAT3; however, pharmacologically targeting SLC1A1 is challenging. We determined a cryo-EM structure of human SLC1A1 bound to compound 3e, a recently described SLC1A1-selective bicyclic imidazo[1,2-]pyridine-3-amine (BIA) inhibitor. 3e binds a membrane-embedded, allosteric pocket accessible only in the apo state, when SLC1A1 is unbound to substrate and sodium. Wedged between the trimerization domain and the substrate-binding transport domain, together with a cholesterol moiety from the lipid bilayer, 3e likely prevents sodium and substrate binding, and blocks SLC1A1\'s elevator-like movements that are essential for transport. Mutations in this pocket abolish 3e binding and counteract 3e\'s cytotoxicity in RCC cells, confirming on-target activity and explaining SLC1A1 selectivity. A structure-guided medicinal chemistry effort yielded two new, SLC1A1-selective BIA derivatives, PBJ1 and PBJ2, with enhanced cytotoxicity resulting from the inhibition of SLC1A1-dependent aspartate, glutamate, and cysteine metabolic pathways.