DPYSL3B is a regulator of chemoresistance via DNA repair and metabolic reprogramming in prostate cancer
DPYSL3B is a regulator of chemoresistance via DNA repair and metabolic reprogramming in prostate cancer
Kaarijarvi, R.; Kaljunen, H.; Itkonen, N.; Bureiko, K.; Ketola, K.
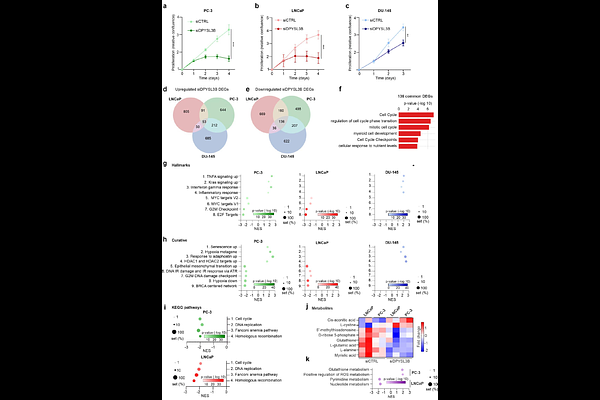
AbstractProstate cancer is among the most common cancers worldwide. Aggressive, treatment-resistant subtypes including androgen receptor (AR) -negative and neuroendocrine prostate cancer, present a significant clinical challenge. Platinum-based chemotherapies, such as carboplatin, remain a treatment option for these subtypes, but their efficacy is limited due to activation of DNA damage response (DDR) pathways. Here, we report that DPYSL3 (CRMP4) is highly expressed in AR-negative prostate cancer, especially in liver metastases. We show that prostate cancer cells express two transcript variants of DPYSL3, DPYSL3A and DPYSL3B. While DPYSL3A has tumor-suppressive roles, the function of DPYSL3B remains unclear. In this study, we demonstrate that high DPYSL3B expression correlates with poor patient survival in prostate cancer. Functional studies revealed that DPYSL3B silencing impairs proliferation and sensitizes prostate cancer cells to carboplatin-induced DNA damage. Gene set enrichment analyses of DPYSL3B-depleted cells showed negative enrichment of pathways related to the cell cycle and DNA repair, including E2F1, AURKA, homologous recombination, and the Fanconi anemia (FA) pathway, consistent with impaired DNA repair capacity. This was accompanied by {gamma}H2AX foci accumulation and apoptosis, as indicated by increased caspase-3/7 activity, particularly under carboplatin exposure. Metabolomic profiling further revealed decreased levels of glutathione and nucleotide precursors, suggesting a role for DPYSL3B in redox homeostasis and nucleotide biosynthesis. Taken together, our transcriptomic, metabolomic, and functional analyses identify DPYSL3B as a regulator of DNA repair and metabolic reprogramming and support its potential as a therapeutic target to overcome chemoresistance in aggressive, treatment-resistant AR-negative prostate cancer.