Control of the signaling of RAS proteins by modulating their palmitoylation
Control of the signaling of RAS proteins by modulating their palmitoylation
Hu, Q.; Zhu, J.; Guo, R.
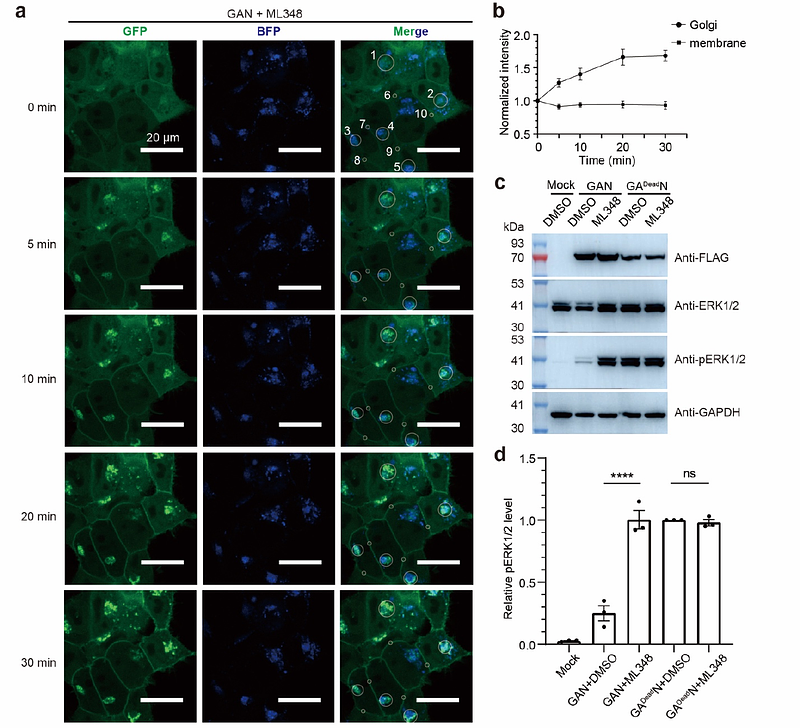
AbstractSmall GTPases, such as the RAS family proteins, are key regulators of cell proliferation and oncogenesis. S-palmitoylation, a reversible post-translational modification catalyzed by ZDHHC enzymes, plays an important role in the regulation of many small GTPases by modulating their subcellular localization. However, development of chemical tools to modulate S-palmitoylation remains challenging due to the redundancy and poor druggability of ZDHHC enzymes. Here, we developed an approach to control the signaling of RAS proteins by modulating their palmitoylation through fusing acyl-protein thioesterase 1 (APT1) to their N-termini. Using in vitro and cell assays, we showed that the subcellular localization and signaling of NRAS in the fusion protein can be reversibly controlled by an APT1 inhibitor, ML348. This approach enables the observation of temporal changes in RAS protein localization, providing a useful tool for studying RAS signaling. Furthermore, similar approaches may be used to control the signaling of other small GTPases.


