Prediction of Gemcitabine sensitivity in resectable Pancreatic Cancer using a Glycation Stress Transcriptomic Signature
Prediction of Gemcitabine sensitivity in resectable Pancreatic Cancer using a Glycation Stress Transcriptomic Signature
Senar, O. A.; Stosic, K.; Bouchart, C.; Navez, J.; Tarfouss, J.; Crake, R.; Verset, L.; Demetter, P.; Mauduit, M.; Conroy, T.; Cros, J.; Nicolle, R.; Detours, V.; Bellahcene, A.; Arsenijevic, T.; Van Laethem, J.-L.
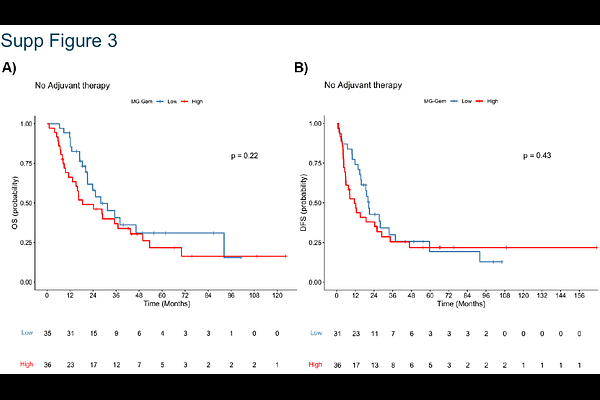
AbstractPurpose Pancreatic ductal adenocarcinoma (PDAC) remains one of the most aggressive malignancies, largely due to chemoresistance arising from prominent cellular heterogeneity and a complex tumor microenvironment. Given this challenge, understanding the mechanisms of driving chemoresistance is essential for guiding our chemotherapeutic choice. Emerging evidence suggests that methylglyoxal (MG), a reactive glycolytic byproduct, may play a role in modulating tumor behavior and therapy response, offering a novel angle for patient stratification. Our recent study indicates that the accumulation of MG in PDAC cells leads to MG-induced cellular stress associated with gemcitabine (GEM) resistance. The primary objective of this study was to identify a predictive transcriptomic signature associated with MG stress in PDAC and compare it to the recently reported GemPred signature. Patients and methods MG stress classification was applied to the Puleo et al. cohort (n=309), enabling differential gene expression analysis and the development of a prognostic 16-gene MG-GEM signature through LASSO Cox regression. The signature was validated for survival prediction and diagnostic performance using cross-validation and ROC analysis. Results The prognostic significance and predictive performance of the signature were validated in both internal and external cohorts, including this from the phase III PRODIGE-24/CCTG PA6 trial, composed of 167 GEM and 183 FOLFIRINOX (mFFX) patients. Importantly, the MG-GEM signature demonstrated independent and complementary predictive value relative to GemPred. Conclusion We present a novel clinically actionable 16-gene transcriptomic signature reflecting MG-induced stress that enables robust stratification of PDAC patients likely to benefit from adjuvant GEM therapy. This model supports the development of personalized treatment strategies in pancreatic cancer.