Pancreatic cancer cachexia is mediated by PTHrP-driven disruption of adipose de novo lipogenesis
Pancreatic cancer cachexia is mediated by PTHrP-driven disruption of adipose de novo lipogenesis
Bhalerao, N. U.; Ogoti, Y.; Peura, J.; Johnson, C.; Chen, Q.; Korobkina, E.; Keller, F.; Wengyn, M.; Norgard, R. J.; Shamber, C.; Klute, K.; DiMaio, D.; Sellin, K.; Grandgenett, P. M.; Li, R.; Hollingsworth, M. A.; GUILHERME, A.; Czech, M.; Kremer, R.; Zhu, L. J.; Watson, E. V.; Ruscetti, M.; Guertin, D.; Pitarresi, J. R.
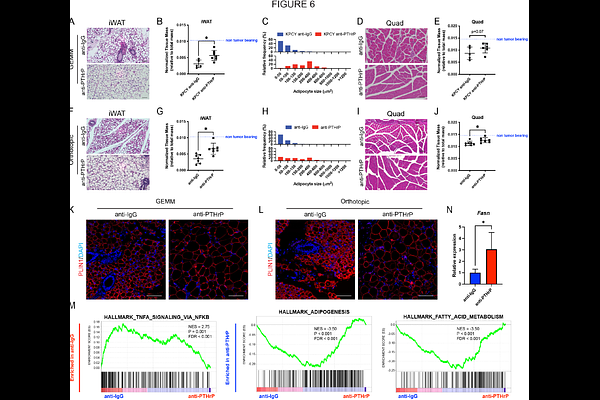
AbstractPancreatic cancer patients have the highest rates and most severe forms of cancer cachexia, yet cachexia etiologies remain largely elusive, leading to a lack of effective intervening therapies. PTHrP has been clinically implicated as a putative regulator of cachexia, with serum PTHrP levels correlating with increased weight loss in PDAC patients. Here we show that cachectic PDAC patients have high expression of tumor PTHrP and use a genetically engineered mouse model to functionally demonstrate that deletion of Pthlh (encoding the PTHrP protein) blocks cachectic wasting, dramatically extending overall survival. The re-expression of PTHrP in lowly cachectic models is sufficient to induce wasting and reduce survival in mice, which is reversed by the conditional deletion of the PTHrP receptor, Pth1r, in adipocytes. Mechanistically, tumor-derived PTHrP suppresses de novo lipogenesis in adipocytes, leading to a molecular rewiring of adipose depots to promote wasting in the cachectic state. Finally, the pharmacological disruption of the PTHrP-PTH1R signaling axis abrogates wasting, highlighting that a targeted disruption of tumor-adipose crosstalk is an effective means to limit cachexia.