TGFβ signaling in cancer-associated fibroblasts drives a hepatic gp130-dependent pro-metastatic inflammatory program in CMS4 colorectal cancer subtype
TGFβ signaling in cancer-associated fibroblasts drives a hepatic gp130-dependent pro-metastatic inflammatory program in CMS4 colorectal cancer subtype
Harryvan, T. J.; Abudukelimu, S.; Stouten, I.; van der Wel, E. J.; Janson, S. G. T.; Lenos, K. J.; Lannagan, T. R. M.; White, M.; Sansom, O. J.; Verdegaal, E. M. E.; Hawinkels, L. J. A. C.
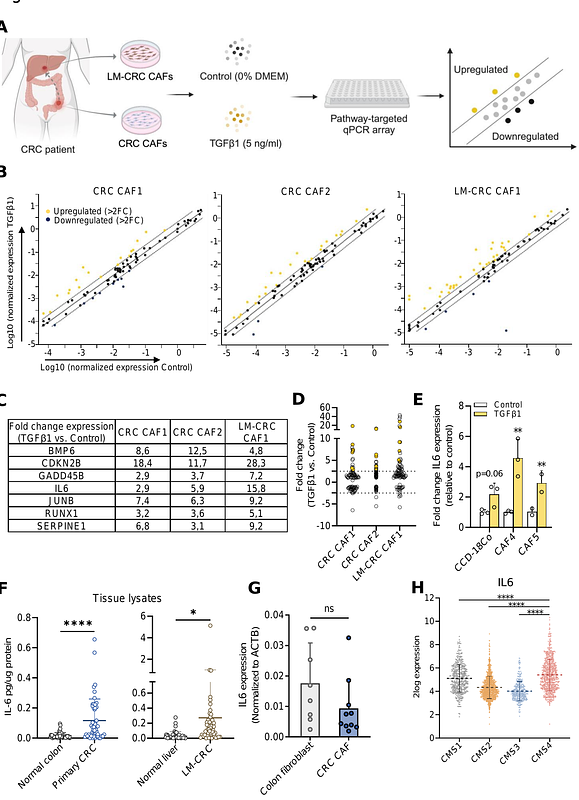
AbstractCurrent molecular classification of colorectal cancer (CRC), the Consensus Molecular Subtypes (CMS), has highlighted the biological heterogeneity of CRC and enables patient stratification based on the molecular subtype of their tumor. The CMS4 subtype shows the worst prognosis and is linked to the highest occurrence of hepatic metastasis but the underlying molecular mechanisms remain unclear. In this study, we show that the molecular features that largely define CMS4 classification, i.e.abundance of cancer-associated fibroblasts (CAFs) in the tumor microenvironment (TME) and active TGF{beta} signaling, converge to promote liver metastasis. Studying TGF{beta} signaling in CRC patient-derived CAFs from the primary tumor revealed that all three TGF{beta} isoforms induce expression of different IL-6 cytokine family members, particularly IL-6 and IL-11. This primary tumor-derived IL-6 and IL-11 in turn induce upregulation of myeloid chemoattractants, including SAA1, in hepatocytes. Chemical inhibition and genetic ablation experiments revealed that gp130, the IL-6 family of cytokine co-receptor, through JAK/STAT signaling is crucial for the induction of neutrophil chemoattractants by hepatocytes and mediates the migration of potential pro-metastatic neutrophils towards the liver. This IL-6 family-JAK/STAT stromal signaling axis is active in both a murine model of CMS4 as well as in human CRC patients in vivo. Combined, our data reveal that TGF{beta} signaling in CAFs actively contributes to the formation of a neutrophil-dependent, pre-metastatic hepatic niche and that this mechanism might play a role in the metastatic phenotype of CMS4 subtype CRC.


