MYC-NFATC2 axis maintains cell cycle and mitochondrial function in AML cells
MYC-NFATC2 axis maintains cell cycle and mitochondrial function in AML cells
Patterson, S. D.; Massett, M. E.; Huang, X.; Jorgensen, H. G.; Michie, A. M.
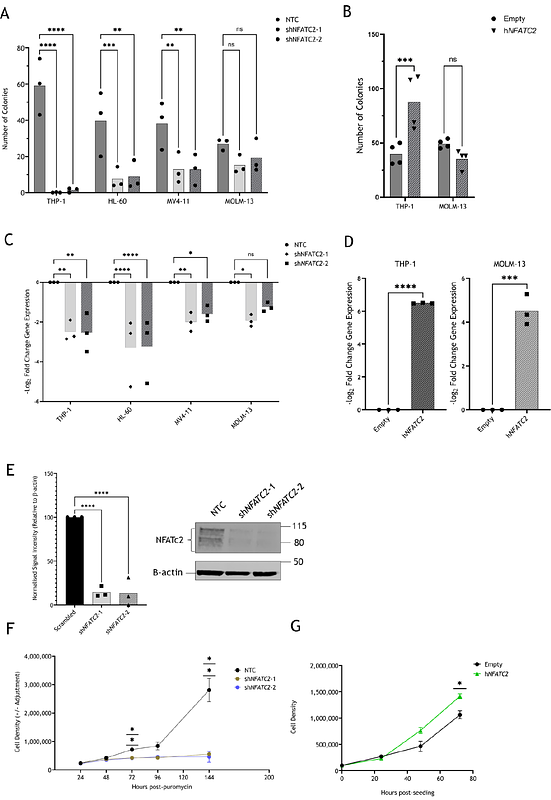
AbstractAcute myeloid leukaemia (AML) is a clonal haematological malignancy affecting the myeloid lineage with generally poor patient outcomes, owing to the lack of targeted therapies. The histone lysine demethylase 4A (KDM4A) has been established as a novel therapeutic target in AML, due to its selective oncogenic role within leukaemic cells. We identify that the transcription factor NFATC2 is a novel binding and transcriptional target of KDM4A in the human AML THP-1 cell line. Further, cytogenetically diverse AML cell lines, including THP-1, were dependent on NFATC2 for colony formation in vitro, highlighting a putative novel mechanism of AML oncogenesis. Our study demonstrates that NFATC2 maintenance of cell cycle progression in human AML cells was driven primarily by CCND1. Through RNA-seq and ChIP-seq, NFATC2 was shown to bind to the promoter region of genes involved in oxidative phosphorylation and subsequently regulate their gene expression in THP-1 cells. Furthermore, our data show that NFATC2 shares transcriptional targets with the transcription factor c-MYC, with MYC knockdown phenocopying NFATC2 knockdown. These data suggest a novel co-ordinated role for NFATC2 and MYC in the maintenance of THP-1 cell function, indicative of a potential means of therapeutic targeting in human AML.