Modulation of ribosomal subunit associations by eIF6 is critical for mitotic exit and cancer progression.
Modulation of ribosomal subunit associations by eIF6 is critical for mitotic exit and cancer progression.
Roshan, P.; Biswas, A.; Anagnos, S.; Luebbers, R.; Harish, K.; Ahmed, S.; Li, M.; Nguyen, N.; Zhou, G.; Tedeschi, F.; Hathuc, V.; Lin, Z.; Hamilton, Z.; Origanti, S. S.
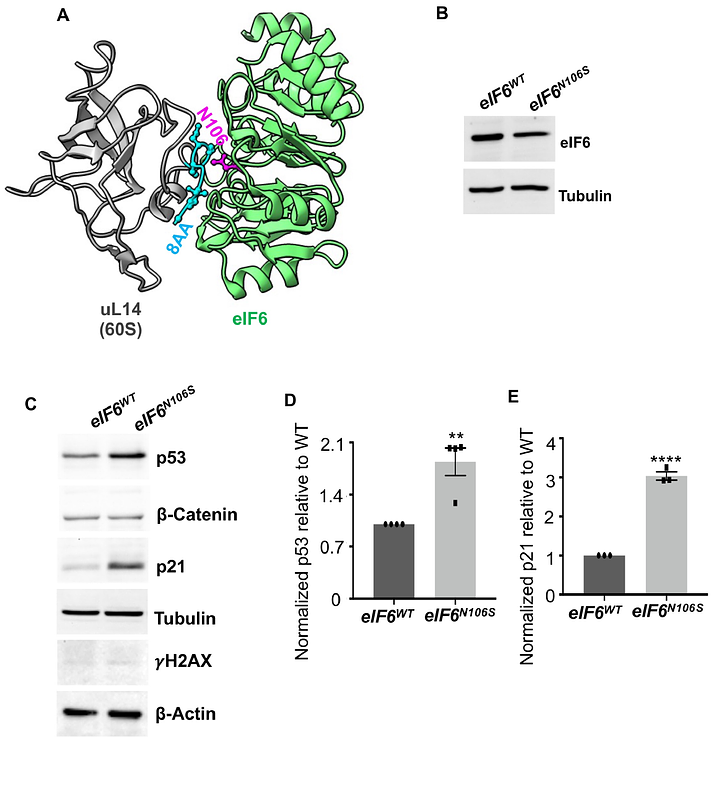
AbstractModerating the pool of active ribosomal subunits is critical for maintaining global translation rates. A factor critical for modulating the pool of 60S ribosomal subunits is eukaryotic translation initiation factor-6 (eIF6). Release of eIF6 from the 60S subunit is important for permitting 60S interactions with 40S to form the 80S monosome. Here, using a mutant of eIF6 (N106S) that interacts poorly with the 60S subunit, we show that the ribosomal subunit associations are deregulated in the absence of eIF6 and lead to an increase in empty 80S ribosomes that decreases global protein synthesis rates. Intriguingly, altering 80S ribosome availability markedly affects the mitotic phase and leads to chromosome segregation defects that induces delays in mitotic exit and mitotic catastrophe. Ribo-Seq analysis of the eIF6-N106S mutant shows a significant downregulation in the translation efficiencies of genes associated with mitosis and cytoskeleton, and specifically transcripts with long 3\' UTRs. eIF6-N106S mutation also markedly affects cancer invasion, and this role is correlated with the overexpression of eIF6 only in high-grade invasive bladder and breast cancers. Thus, this study highlights the importance of therapeutically targeting the eIF6-60S interaction interface in cancers and the importance of modulating active 80S ribosome availability for mitotic translation and mitotic exit.


