Glycodiversification of gentamicins through in vivo glycosyltransferase swapping enabled the creation of novel hybrid aminoglycoside antibiotics with potent activity and low ototoxicity
Glycodiversification of gentamicins through in vivo glycosyltransferase swapping enabled the creation of novel hybrid aminoglycoside antibiotics with potent activity and low ototoxicity
Jian, X.; Wang, C.; Wu, S.; Sun, G.; Huang, C.; Qiu, C.; Liu, Y.; Leadlay, P. F.; Liu, D.; Deng, Z.; Zhou, F.; Sun, Y.
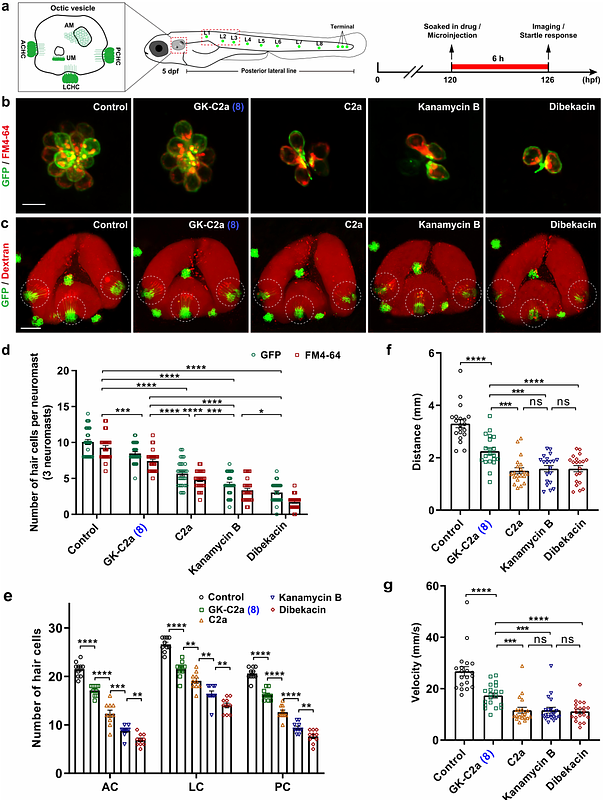
AbstractAminoglycosides (AGs) are a class of potent antibiotics with a broad spectrum of activity. However, their use is limited by safety concerns associated with nephrotoxicity and ototoxicity, as well as drug resistance. To address these issues, semi-synthetic approaches for modifying natural AGs have successfully generated new generations of AGs, however, with limited types of modification due to significant challenges in synthesis. This study explores a novel approach that harness the bacterial biosynthetic machinery of gentamicins and kanamycins to create hybrid AGs, installing extensive natural modifications from gentamicins onto kanamycins. This was achieved by glycodiversification of gentamicins via swapping the glycosyltransferase (GT) in their producer with the GT from kanamycins biosynthetic pathway and resulted in the creation of a series of novel AGs with combined structural features of two, therefore referred to as genkamicins (GKs). The manipulation of the hybrid metabolic pathway enabled the target accumulation of different GK species and the successful isolation and characterization of six GK components. These compounds display retained antimicrobial activity against a panel of World Health Organization (WHO) critical priority pathogens, and GK-C2a, in particular, demonstrates low ototoxicity compared to clinical drugs in zebrafish embryos. This study provides a new strategy for diversifying the structure of AGs and a potential avenue for developing less toxic AG drugs to combat infectious diseases.