Decoding menopause-induced tissue fibrosis using pan-tissue network inference
Decoding menopause-induced tissue fibrosis using pan-tissue network inference
Iijima, H.; Yamashita, A.; Galloway, J. L.; Vo, N.; Choi, H. S.; Ambrosio, F.
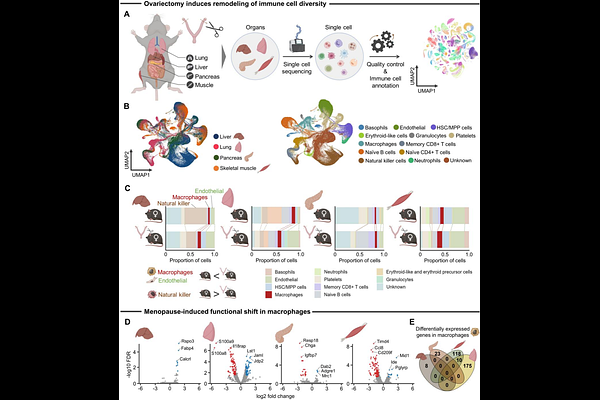
AbstractMenopause drives fibrotic remodeling and consequent tissue dysfunction across multiple organs, yet the tissue-conserved versus tissue-specific mechanisms underlying this phenomenon remain poorly defined. Here, we employed a network inference framework to uncover how menopause triggers coordinated shifts in intercellular signaling cascades that promote fibrosis. We leveraged publicly archived single-cell RNA-sequencing data from the liver, lung, pancreas, and skeletal muscle of ovariectomized and control mice. Given the central role of immune cells in orchestrating inflammation and fibrosis, we focused our analysis on immune cell populations. Using canonical markers of immune cells, we annotated major cell types and reconstructed cell-cell interaction networks to map transcriptional responses to hormonal shifts. Network analysis revealed pervasive reshaping of intercellular signaling common to all tissues evaluated in the setting of menopause. Within this immune-centered framework, we found that estrogen-responsive macrophages consistently function as major signaling hubs across all tissues evaluated, exhibiting extensive interactions with myofibroblasts, key drivers of extracellular matrix production and fibrotic remodeling. Notably, these shared signaling patterns were not detectable using conventional differential gene expression analysis, which revealed minimal overlap in gene-level responses in macrophages across tissues. In addition to conserved patterns, we identified tissue-specific interaction networks that reflect unique immune adaptations to hormonal loss. As an example, natural killer cells acted as a signaling hub in muscle-specific patterns, suggesting their direct contribution to menopausal skeletal muscle adaptation. Tissue-specific patterning was also evident in the liver, lung, and pancreas, where other immune cell types, such as CD8+ T cells and endothelial cells, functioned as prominent signaling hubs, indicating diverse remodeling of the immune microenvironment. The network approach introduced here represents a systems-level framework for mapping multicellular network rewiring following hormonal depletion and highlights conserved immune-stromal modules as potential therapeutic targets to prevent menopause-associated dysfunction.