Automodification of N-terminal serine residues in PARP2 impacts PARP2 release from DNA damage sites
Automodification of N-terminal serine residues in PARP2 impacts PARP2 release from DNA damage sites
Dhakar, S. S.; Galera-Prat, A.; Cai, J.; Preisser, J.; Kiema, T.-R.; Ladurner, A.; Lehtiö, L.
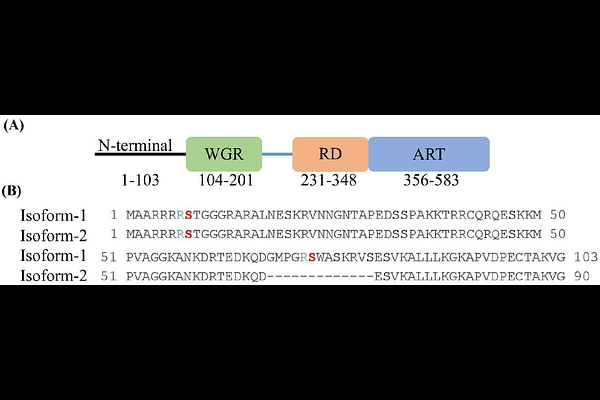
AbstractADP-ribosyltransferases PARP1 and PARP2 are involved in DNA repair mechanisms and play a major role in detecting DNA damage. PARP1/2 enzymes transfer the ADP-ribosyl moiety from NAD+ to DNA damage proteins, histones and to itself, activating the DNA repair cascade. Recent studies have shown that Histone PARylation Factor (HPF1) forms a joint active site with the catalytic domain of PARP1/2 and alters their target modification site from glutamate/aspartate towards serine. In this work, we have identified key serine residues within the N-terminus of full-length PARP2 that are the main targets of PARP1/2 automodification. We demonstrated this using site-directed mutagenesis, gel-based PARylation assays of automodification reactions, and by measuring the release of PARP2 from the DNA damage site using a fluorescence polarization assay. We show that in the presence of HPF1, PARP2 serine 8 and serine 73 are predominantly ADP-ribosylated and serine 8, which is present in both human PARP2 isoforms, is the major site for PARP2 automodification. Our results provide insight into the mechanistic role of the N-terminus of PARP2 in the PARylation-dependent release of PARP2 from DNA damage sites.