PGM1 deficiency disrupts sarcomere and mitochondrial function in a stem-cell cardiomyocyte model
PGM1 deficiency disrupts sarcomere and mitochondrial function in a stem-cell cardiomyocyte model
Radenkovic, S.; Preston, G.; Budhraja, R.; Muffels, I.; Ligezka, A. N.; Hrstka, R.; Staff, N. P.; Balakrishan, B.; Shah, R.; Verberkmoes, S.; Shammas, I.; Bosnyak, I.; Stiers, K. M.; Lai, K.; Beamer, L. J.; Pandey, A.; Morava, E.; Kozicz, T.
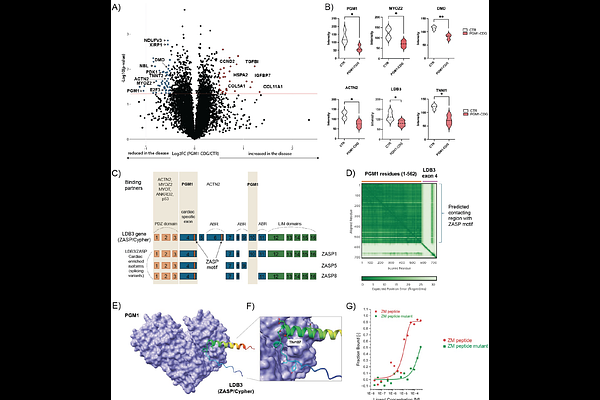
AbstractBackground Phosphoglucomutase-1 (PGM1) plays a pivotal role in glycolysis, glycogen metabolism, and glycosylation. Pathogenic variants in PGM1 cause PGM1-congenital disorder of glycosylation (PGM1-CDG), a multisystem disorder with cardiac involvement. While glycosylation abnormalities in PGM1-CDG are treatable with galactose, cardiomyopathy does not improve suggesting a glycosylation-independent pathomechanism. Recently, mitochondrial abnormalities have been shown in a heart of a PGM1-deficicient patient and PGM1-mouse model. In addition, PGM1 has been associated with LDB3 (ZASP/Cypher), a sarcomeric Z-disk protein also associated with cardiomyopathy. However, the cardiac-specific role of PGM1 remains poorly understood, and targeted therapies for PGM1-related cardiomyopathy are currently lacking. Methods Induced pluripotent stem cell-derived cardiomyocytes (iCMs) were generated from PGM1-deficient patient fibroblasts. Multielectrode array (MEA) recordings, untargeted (glyco)proteomics, and pathway analysis were performed to assess functional and molecular changes. Key findings were validated using tracer metabolomics and mitochondrial respiration assays. Results PGM1-deficient iCMs exhibited reduced beating frequency, impaired contractility, and prolonged contraction kinetics. Proteomic analyses revealed depletion of Z-disk components, including LDB3. AlphaFold3 structural modeling predicted a direct interaction between PGM1 and LDB3, implicating PGM1 in Z-disk integrity, which was confirmed in vitro. In addition, mitochondrial proteins were severely depleted, prompting us to investigate mitochondrial function. Functional validation confirmed extensive metabolic rewiring, energy depletion, and severely impaired mitochondrial respiration. Finally, the in silico drug repurposing identified possible therapeutic options that could target PGM1-deficient cardiomyopathy. Conclusion PGM1 is a key regulator of cardiomyocyte function, linking sarcomeric Z-disk integrity with mitochondrial metabolism. These mechanistic insights offer a foundation for developing targeted therapies for PGM1-CDG and potentially other cardiomyopathies involving Z-disk dysfunction.