G51D mutation of the endogenous rat Snca gene disrupts synaptic localisation of α-synuclein priming for Lewy-like pathology
G51D mutation of the endogenous rat Snca gene disrupts synaptic localisation of α-synuclein priming for Lewy-like pathology
West, S.; Natalwala, A.; Dolt, K. S.; Lamont, D. J.; McMillan, M.; Luk, K.; Mashimo, T.; Kunath, T.
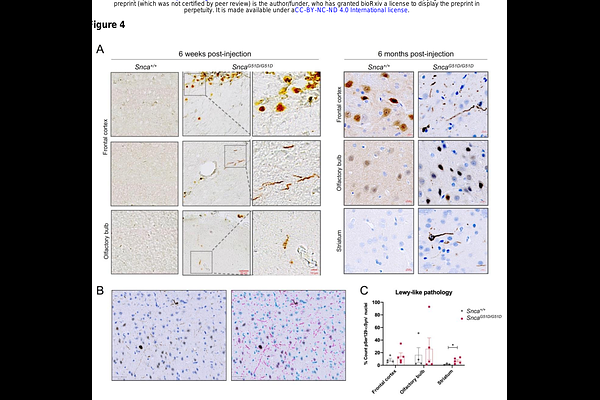
AbstractPoint mutations in the SNCA gene, encoding alpha-synuclein (aSyn), are a known cause of familial Parkinsons disease. The G51D mutation causes early onset neurodegeneration with complex pathology. We used CRISPR/Cas9 in rats to introduce the G51D mutation into the endogenous Snca gene. Co-localisation immunostaining studies with synaptic proteins showed that aSynG51D protein is no longer efficiently localised to synapses. Furthermore, biochemical isolation of synaptosomes from rat cortex demonstrated a significant depletion of aSyn in SncaG51D/+ and SncaG51D/G51D rats. Unbiased proteomic investigation of the cortex identified significant synaptic dysregulation in SncaG51D/G51D animals. Finally, we compared the propensity for Lewy-like pathology of Snca+/+ and SncaG51D/G51D rats by stereotaxically delivering aSyn pre-formed fibrils (PFFs) into the pre-frontal cortex. At an early time-point, 6 weeks post-injection, we observed discrete Lewy-like structures positive for phosphoserine-129-aSyn (pS129-aSyn) only in SncaG51D/G51D brains. At 26 weeks post-injection of PFFs SncaG51D/G51D brains exhibited intense, discrete pS129- aSyn-positive structures, while Snca+/+ brains exhibited diffuse pS129-aSyn immunostaining. Quantification of discrete pS129-aSyn-positive structures revealed the striatum of SncaG51D/G51D rats had significantly more Lewy-like pathology than Snca+/+ rats. In summary, this novel SncaG51D rat model exhibits molecular characteristics of early synaptic dysfunction and is primed for aSyn pathology.