A Protein Disulfide Isomerase Coordinates Redox Homeostasis and ER Calcium Regulation for Optimal Lytic Cycle Progression in Toxoplasma gondii
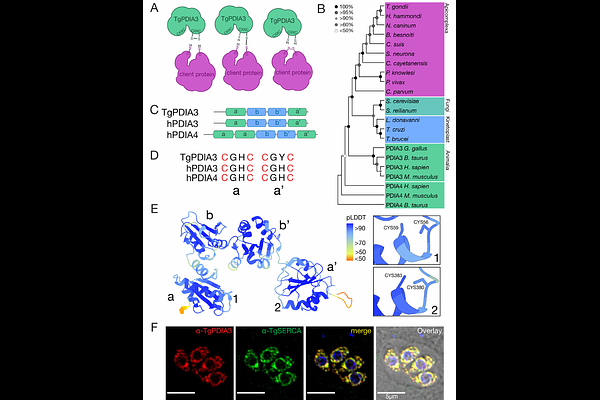
A Protein Disulfide Isomerase Coordinates Redox Homeostasis and ER Calcium Regulation for Optimal Lytic Cycle Progression in Toxoplasma gondii
Moen, K. E.; Moreno, S. N. J.
AbstractThe endoplasmic reticulum (ER) maintains an oxidative environment that facilitates disulfide bond formation, a critical process for proper protein folding. Protein disulfide isomerases (PDIs) are ER resident enzymes that facilitate the formation, breakage, and rearrangement of disulfide bonds between cysteine residues, thereby stabilizing protein structures. Although PDIs are functionally diverse, they all contain at least 1 thioredoxin-like domain and mediate disulfide exchange through their conserved CXXC motifs. The Apicomplexan parasite, Toxoplasma gondii, infects approximately one third of the world population, posing a significant risk to immunosuppressed individuals and unborn fetuses. The fast-replicating tachyzoite form engages in a lytic cycle, causing host tissue damage and contributing to pathogenesis. While approximately 26 PDIs are predicted to be present in T. gondii, their specific roles remain largely unexplored. In this study, we investigate TgPDIA3, a T. gondii PDI localized to the ER, along with several of its interacting protein substrates. We explore its role in ER redox activity and calcium sequestration and assess how these functions contribute to the parasite lytic cycle.