Single-vesicle tracking of α-synuclein oligomers reveals pore formation by a three-stage model modulated by charge, curvature, lipids and ligands
Single-vesicle tracking of α-synuclein oligomers reveals pore formation by a three-stage model modulated by charge, curvature, lipids and ligands
Malle, M. G.; Otzen, D. E.; Brochner, B. V.; Zhang, X.; Nielsen, J.; Kjems, J.
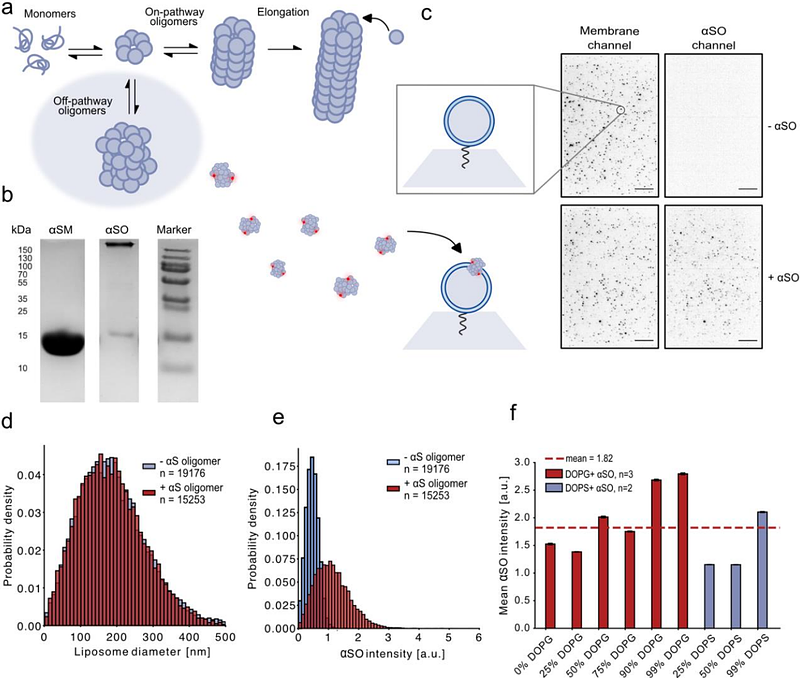
AbstractNeurodegenerative disorders like Parkinson\'s disease (PD) pose significant health challenges. A major hallmark is the aggregation of -synuclein into toxic oligomers (SO) and fibrils. While many efforts focus on slowing disease progression, the molecular origins and mechanisms of SO toxicity remain poorly understood, particularly its proposed link to membrane disruption. To address this, we have developed a single-vesicle analysis platform for direct and real-time measurements of SO and membrane interaction. This allows us to show real-time translocation of dyes through SO pores with single-particle resolution and single-channel electrical recordings for analyzing pore formation in planar lipid bilayers. Across methods, our data reveal evidence for a three-stage model for SO and membrane interactions with initial membrane recruitment followed by partial pore insertion and subsequent full pore formation. Strikingly, while SO recruitment was found to favor curved membranes, pore formation occurred more efficiently in less curved membranes, hence recruitment is divorced from a membrane charge-promoted reorientation and pore integration. Single SO pore formations are undergoing multiple translocation steps hence pore formation is highly dynamic cycling back and forth from partial insertion to full pore formation. The dynamic nature of pore formation can be modulated by lipid charge, lipid headgroup class, and ligand binding. Our findings suggest that increased dynamic pore formation could implies increased membrane toxicity. Evidence for the three-stage model is important for future targeting strategies for blocking SO mitigated PD-related cellular dysfunction and we envision the single-vesicle assay enables screening for ligands modulating the pore formation.