Bacterial motility depends on a critical flagellum length and energy-optimised assembly
Bacterial motility depends on a critical flagellum length and energy-optimised assembly
Halte, M.; Popp, P. F.; Hathcock, D.; Severn, J.; Fischer, S.; Goosmann, C.; Ducret, A.; Charpentier, E.; Tu, Y.; Lauga, E.; Erhardt, M.; Renault, T. T.
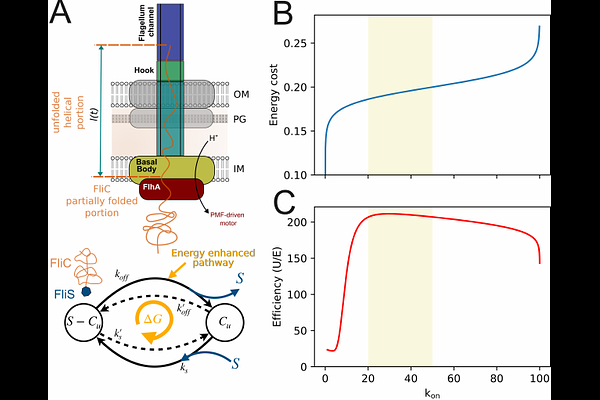
AbstractThe flagellum is the most complex macromolecular structure known in bacteria and comprised of around two dozen distinct proteins. The main building block of the long, external flagellar filament, flagellin, is secreted through the flagellar type-III secretion system at a remarkable rate of several tens of thousands amino acids per second, significantly surpassing the rates achieved by other pore-based protein secretion systems. The evolutionary implications and potential benefits of this high secretion rate for flagellum assembly and function, however, have remained elusive. In this study, we provide both experimental and theoretical evidence that the flagellar secretion rate has been evolutionarily optimized to facilitate rapid and efficient construction of a functional flagellum. By synchronizing flagellar assembly, we found that a minimal filament length of 2.5 um was required for swimming motility. Biophysical modelling revealed that this minimal filament length threshold resulted from an elasto-hydrodynamic instability of the whole swimming cell, dependent on the filament length. Furthermore, we developed a stepwise filament labeling method combined with electron microscopy visualization to validate predicted flagellin secretion rates of up to 10,000 amino acids per second. A biophysical model of flagellum growth demonstrates that the observed high flagellin secretion rate efficiently balances filament elongation and energy consumption, thereby enabling motility in the shortest amount of time. Taken together, these insights underscore the evolutionary pressures that have shaped the development and optimization of the flagellum and type-III secretion system, illuminating the intricate interplay between functionality and efficiency in assembly of large macromolecular structures.