Tau P301L mutation promotes core 4R tauopathy fibril fold through near-surface water structuring and conformational rearrangement
Tau P301L mutation promotes core 4R tauopathy fibril fold through near-surface water structuring and conformational rearrangement
Vigers, M.; Lobo, S.; Najafi, S.; Dubose, A.; Tsay, K.; Ganguly, P.; Longhini, A. P.; Jin, Y.; Buratto, S. K.; Kosik, K. S.; Shell, M. S.; Shea, J.-E.; Han, S.
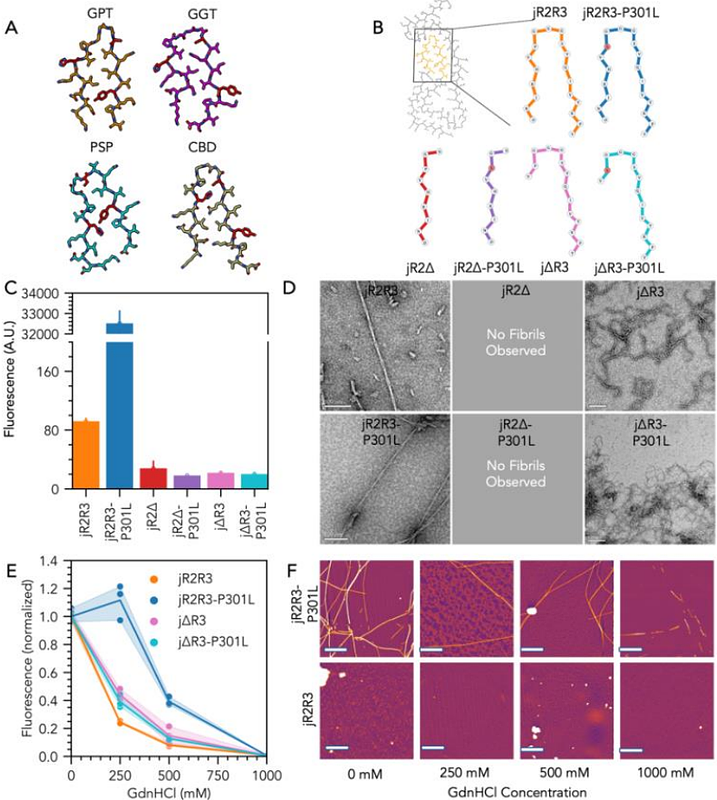
AbstractTau forms toxic fibrillar aggregates in a family of neurodegenerative diseases known as tauopathies. The faithful replication of tauopathy-specific fibril structures is a critical gap for developing diagnostic and therapeutic tools. This study debuts a strategy of identifying a critical segment of tau that forms a folding motif that is characteristic of a family of tauopathies and isolating it as a standalone peptide that form seeding-competent fibrils. The 19-residue jR2R3 peptide (295-313) spanning the R2/R3 splice junction of tau, in the presence of P301L, forms seeding-competent amyloid fibrils. This tau fragment contains the hydrophobic VQIVYK hexapeptide that is part of the core of every pathological tau fibril structure solved to-date and an intramolecular counter-strand that stabilizes the strand-loop-strand (SLS) motif observed in 4R tauopathy fibrils. This study shows that P301L exhibits a duality of effects: it lowers the barrier for the peptide to adopt aggregation-prone conformations and enhances the local structuring of water around the mutation site that facilitates site-specific dewetting and in-register stacking of tau to form cross {beta}-sheets. We solve a 3 [A] cryo-EM structure of jR2R3-P301L fibrils with a pseudo 21 screw symmetry in which each half of the fibril\'s cross-section contains two jR2R3-P301L peptides. One chain adopts a SLS fold found in 4R tauopathies that is stabilized by a second chain wrapping around the SLS fold, reminiscent of the 3-fold and 4-fold structures observed in 4R tauopathies. These jR2R3-P301L fibrils are able to template full-length tau in a prion-like fashion.