Mapping allosteric rewiring in related protein structures from collections of crystallographic multiconformer models
Mapping allosteric rewiring in related protein structures from collections of crystallographic multiconformer models
Raju, A.; Sharma, S.; Riley, B. T.; Djuraev, S.; Tan, Y.; Kim, M.; Mahmud, T.; Keedy, D. A.
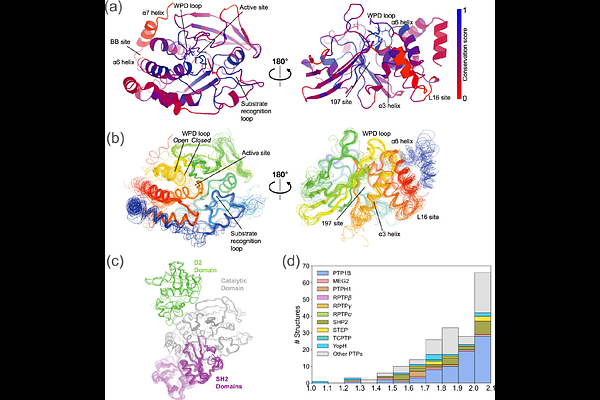
AbstractHow do related proteins with a common fold perform diverse biological functions? Although the average structure may be similar, structural excursions from this average may differ, giving rise to allosteric rewiring that enables differential activity and regulation. However, this idea has been difficult to test in detail. Here we used the qFit algorithm to model \"hidden\" alternate conformations from electron density maps for an entire protein family, the Protein Tyrosine Phosphatases (PTPs), spanning 26 enzymes and 221 structures. To interrogate these multiconformer models, we developed a new algorithm, Residue Interaction Networks From Alternate conformations In RElated structures (RINFAIRE), that calculates networks of interactions between flexible residues and quantitatively compares them. We show that PTPs share a common allosteric network which rewires dynamically in response to catalytic loop motions or active-site vs. allosteric ligand binding, but also that individual PTPs have unique allosteric signatures. As experimental validation, we show that targeted mutations at residues with varying sequence conservation but high network connectivity modulate enzyme catalysis, including a surprising enhancement of activity. Overall, our work provides new tools for understanding how evolution has recycled modular macromolecular building blocks to diversify biological function. RINFAIRE is available at https://github.com/keedylab/rinfaire.