Preprint: Triggered sequential viral-transduction from collagen-based scaffolds for tissue regeneration
Preprint: Triggered sequential viral-transduction from collagen-based scaffolds for tissue regeneration
Amante, J. J.; Twombly, B.; Thotathil, N.; Kearney, C. J.
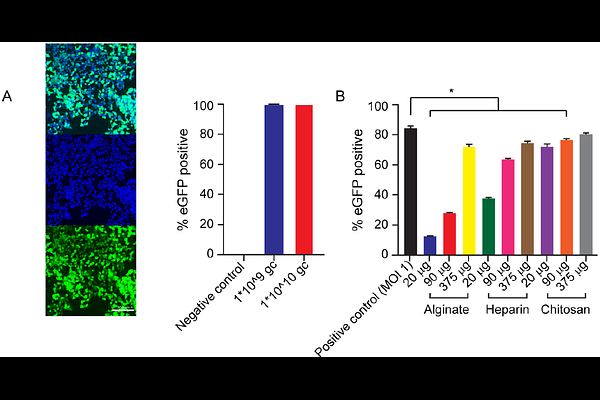
AbstractChronic wounds are a major healthcare issue that are recalcitrant to many traditional treatments. Increasingly, tissue engineering scaffolds are being developed and translated to promote their healing. To control signaling in the wound environment, gene therapy approaches are being explored, with adeno-associated virus (AAV) becoming increasingly popular. One critical challenge in chronic wound healing is that the wounds do not progress through the typical wound healing cascade, with signaling getting stuck in the inflammatory/immature tissue formation phase. This motivated us to develop a system capable of triggered sequential release of viral vectors to drive coordinated signaling. By housing this system within a collagen-glycosaminoglycan (GAG) scaffold, we aim to provide a proven extracellular matrix template as well as the correct signaling profile for closure of chronic wounds. Our system consists of two alginate pockets within the collagen-GAG scaffold, which we use to control the release of AAV. The first pocket allows diffusion of one AAV therapeutic and the second pocket can be ultrasound-triggered using low-frequency stimulation to release the second therapeutic. Initially, we developed and characterized the system using a reporter AAV. At our high AAV loading, we got sustained release and GFP expression in HEK293T cells over 9 days from our system in vitro, but lower loading had minimal transduction. When this lower group was triggered with ultrasound, cells were successfully transduced. Finally, we demonstrated sequential release of AAV encoding clinically-relevant genes for angiogenesis. This system has the potential for broad applicability as it can be readily adapted to mimic a range of biological pathways.