Embryonic lymphocytes contribute to a genetic form of autoimmune inflammation
Embryonic lymphocytes contribute to a genetic form of autoimmune inflammation
Cascione, S.; Fontana, E.; Scarfo, R.; Rigoni, R.; Capo, V.; Draghici, E.; Dobbs, K.; Villa, A.; Notarangelo, L. D.; Ditadi, A.
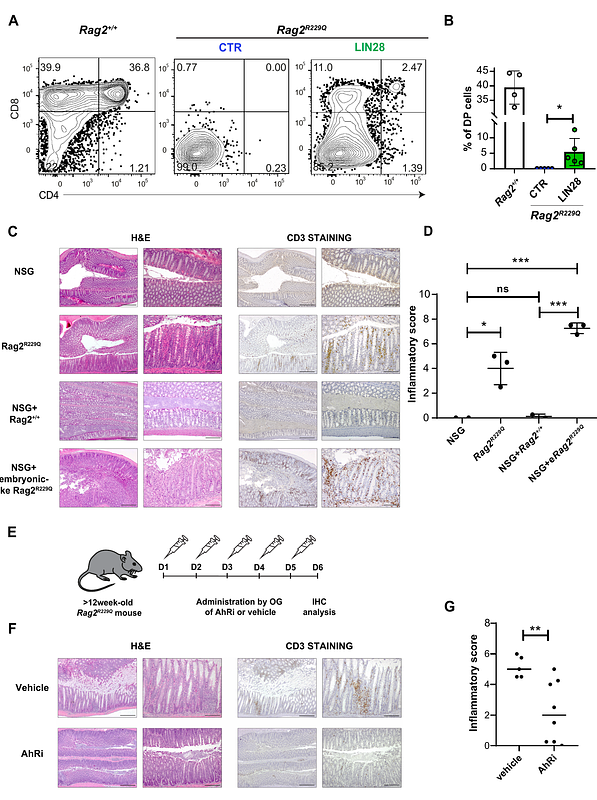
AbstractOmenn Syndrome (OS) is a rare hematological disorder, caused by hypomorphic mutations in genes involved in B-/T-cell receptor (BCR/TCR) rearrangement that result in impaired lymphocyte development and immunodeficiency. Notwithstanding, few T-cell clones enriched in self-reactive specificities expand in peripheral tissues, where they trigger severe inflammation and autoimmune reactions. Interestingly, residual OS lymphocytes display characteristics proper of embryonic lymphocytes that emerge before, and independently from, hematopoietic stem cells (HSCs). This prompted us to hypothesize whether OS autoreactive T-cells are generated in the embryo independently from HSCs. Here we show that in the Rag2R229Q/R229Q OS mouse model, embryonic but not adult bone marrow-derived hematopoietic progenitors can generate T-cells. T-lymphopoiesis can be rescued in adult OS blood progenitors via their Lin28-mediated reprogramming to an embryonic-like state. Remarkably, when transplanted in immunodeficient mice, embryonic-like OS progenitors trigger tissue morphological alterations and inflammation in the large intestine of the recipients, recapitulating the typical OS inflammatory phenotype. Our study describes the previously unappreciated contribution of embryonic progenitors to the pool of autoreactive infilitrating T-cells, providing a novel platform for both the detailed study of human autoimmune disorders and the design of more targeted therapies.