Accessing anti-HIV activity through the attenuation of USP18 activity: novel insights from molecular dynamic simulations, free-energy profiling, and multi-cellular inhibition assays
Accessing anti-HIV activity through the attenuation of USP18 activity: novel insights from molecular dynamic simulations, free-energy profiling, and multi-cellular inhibition assays
Sigauke, L. T.; Bvunzawabaya, J.; Le Bury, G.; Dziwornu, G. A.; Boliar, S.; Gludish, D. W.; Russell, D. G.; Govender, K.; Mugumbate, G.; Chigorimbo-Murefu, N.
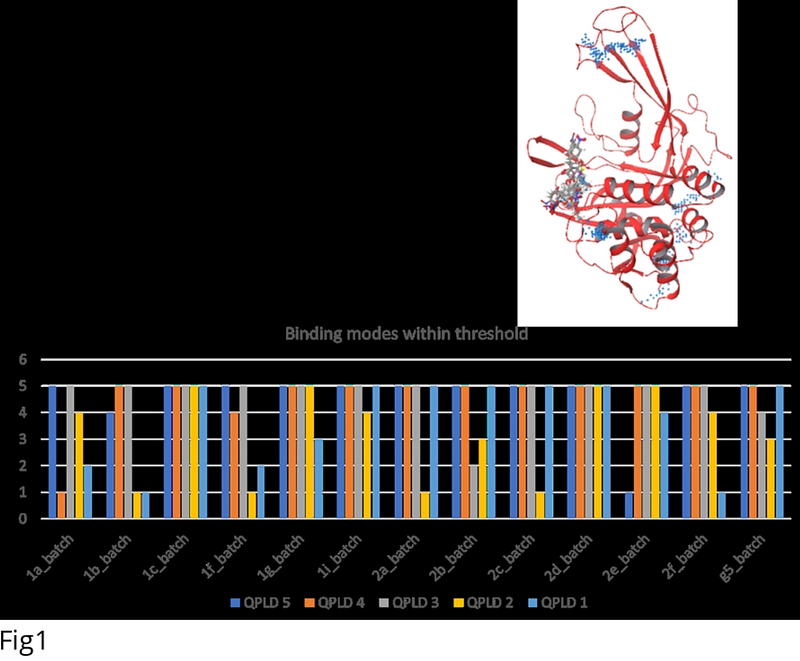
AbstractThe feasibility of achieving anti-HIV activity from the attenuation of USP18 activity was explored for the first time. A cheminformatic survey demonstrated that the current known USP18 isopeptidase inhibitors are derivatives of a bis-aryl pyranone scaffold that possesses undesirable toxicity profiles. Molecular modelling approaches applied to these active bis-aryl pyranones isolated the likely mechanism that perturbs the isopeptidase activity of USP18. Molecular dynamic simulations and free-energy profiling showed that induced-fit effects on the catalytic triad and the IBB-1 domain residues of USP18 drive a reversible non-competitive isopeptidase inhibition mechanism. Proof-of-concept multi-cellular HIV inhibition assays demonstrate the utility of achieving anti-HIV-1 activity from attenuating the activity of USP18 using small molecules. This study motivates for the pursuit of scaffolds that target the allosteric site of USP18, fine-tuning the IFN response as a strategy to enhance the natural control mechanisms that lead to an antiviral state potentially curing viral infection.


