The decameric repeat (DR) of PSGL-1 functions as a basic antiviral unit in restricting HIV-1 infectivity
The decameric repeat (DR) of PSGL-1 functions as a basic antiviral unit in restricting HIV-1 infectivity
Han, Y.; Sealey, L.; Fu, Y.; Delfing, B. M.; Lockhart, C.; Chilin, L.; Tiwari, S.; Jafri, M. S.; Klimov, D.; Wu, Y.
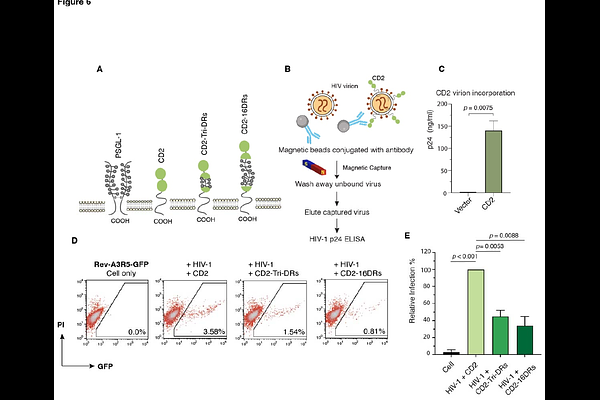
AbstractPSGL-1 (P-selectin glycoprotein ligand-1) is a dimeric, mucin-like surface glycoprotein that interacts with selectins on the endothelium, facilitating leukocyte tethering and rolling during transmigration. Previous studies have demonstrated that PSGL-1 acts as an HIV restriction factor, blocking HIV infectivity by hindering particle attachment to target cells. A large part of PSGL-1\'s extracellular region consists of a mucin-like domain, characterized by decameric repeats (DR), which comprise 14 to 16 DR tandems. Each DR is composed of 10 consensus amino acids (-A-T/M-E-A-Q-T-T-X-P/L-A/T-) and is notably rich in O-glycosylated threonines (30%) and prolines (10%). A proposed function of the DR is to elongate the protein backbone needed for selectin binding. However, the precise role of DR in PSGL-1\'s antiviral mechanisms has yet to be fully elucidated. In this study, we performed DR deletion mutagenesis and molecular modeling, systematically deleting from one DR to all DRs, and quantified their effects on PSGL-1\'s antiviral functions. Here, we demonstrate that DR is necessary for PSGL-1\'s antiviral activity. Deleting DR did not affect virion incorporation of PSGL-1, but diminished PSGL-1\'s ability to hinder virion attachment to target cells. We also discovered that individual single DR mutants retained 3.5 to 18% of the antiviral activity exhibited by full-length PSGL-1, and the basic antiviral activity of DR is cumulative; increasing the number of DRs correlates with heightened antiviral activity. Additionally, the intrinsic anti-HIV capability of DR was found to be transferrable; inserting the DR domain into the extracellular Ig-like domain of CD2 permitted the hybrid CD2-DR molecules to partially acquire the anti-HIV properties of PSGL-1. Further molecular modeling utilizing all-atom replica exchange molecular dynamics simulations highlighted a structure-function relationship between the anti-HIV potency of DR and the elongation of the DR backbone, as measured by DR backbone dihedral angles ({phi}) and the radial distributions of peptide atom number density (r max). These results collectively suggest that DR serves as an essential antiviral unit within the framework of PSGL-1\'s restriction mechanism against HIV.