Microglia Depletion Reduces Human Neuronal APOE4-Driven Pathologies in a Chimeric Alzheimer's Disease Model
Microglia Depletion Reduces Human Neuronal APOE4-Driven Pathologies in a Chimeric Alzheimer's Disease Model
Rao, A.; Chen, N.; Kim, M. J.; Blumenfeld, J.; Yip, O.; Hao, Y.; Liang, Z.; Nelson, M. R.; Koutsodendris, N.; Grone, B.; Ding, L.; Yoon, S. Y.; Arriola, P.; Huang, Y.
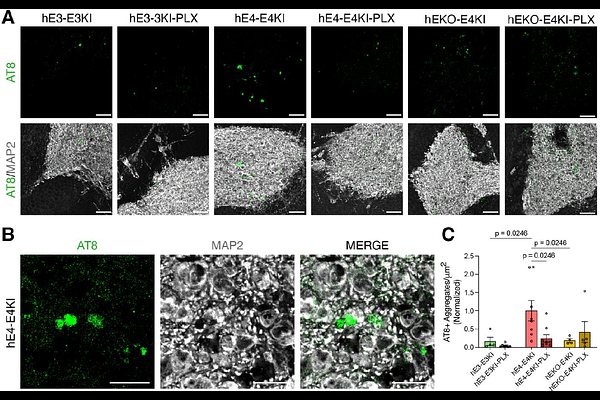
AbstractDespite strong evidence supporting the involvement of both apolipoprotein E4 (APOE4) and microglia in Alzheimer\'s Disease (AD) pathogenesis, the effects of microglia on neuronal APOE4-driven AD pathogenesis remain elusive. Here, we examined such effects utilizing microglial depletion in a chimeric model with human neurons in mouse hippocampus. Specifically, we transplanted homozygous APOE4, isogenic APOE3, and APOE-knockout (APOE-KO) induced pluripotent stem cell (iPSC)-derived human neurons into the hippocampus of human APOE3 or APOE4 knock-in mice, and depleted microglia in half the chimeric mice. We found that both neuronal APOE and microglial presence were important for the formation of A{beta} and tau pathologies in an APOE isoform-dependent manner (APOE4 > APOE3). Single-cell RNA-sequencing analysis identified two pro-inflammatory microglial subtypes with high MHC-II gene expression that are enriched in chimeric mice with human APOE4 neuron transplants. These findings highlight the concerted roles of neuronal APOE, especially APOE4, and microglia in AD pathogenesis.