Salmonella SteD and mammalian SUSD6 use TMEM127 to co-disinhibit the WWP2 E3 ubiquitin ligase
Salmonella SteD and mammalian SUSD6 use TMEM127 to co-disinhibit the WWP2 E3 ubiquitin ligase
Blundell, S. V.; Liu, M.; Tocci, R.; Majstorovic, A.; Tsang, S.; Holden, D. W.
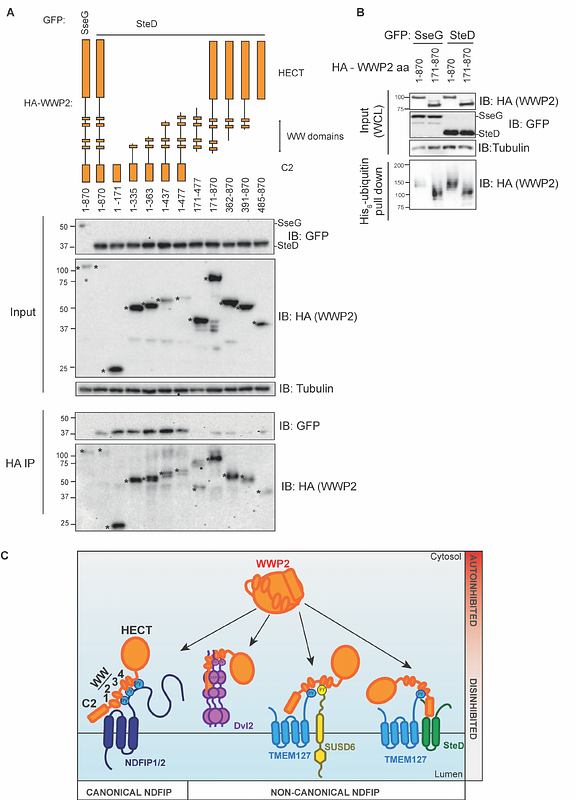
AbstractThe NEDD4-like E3 ubiquitin ligase, WWP2, is involved in a range of host processes from cell differentiation to T cell immunity. Ligase activity is tightly regulated with WWP2 being held in an autoinhibited state. Binding of a PY motif containing adaptor, an Ndfip, via the WW domains of NEDD4-like E3 ubiquitin ligases leads to their disinhibition. Here, we show that the canonical Ndfip, NDFIP2, requires multiple PY motifs for interaction with and activation of WWP2. In contrast, the single PY-motif containing Ndfips TMEM127 and SUSD6 function as a co-disinhibitory pair. TMEM127 and the Salmonella protein SteD also function as a co-disinhibitory pair. However, SteD interacts with a different region of the WWP2, the C2 domain, and this interaction results in disinhibition of WWP2. These findings demonstrate a range of ways that Ndfips can disinhibit WWP2. To our knowledge, these are the first examples of two Ndfips functioning as co-disinhibitory pairs, and of a bacterial effector that disinhibits an E3 ubiquitin ligase.