Regulatory T cell stability determines the efficiency of bile duct regeneration during cholangitis.
Regulatory T cell stability determines the efficiency of bile duct regeneration during cholangitis.
Kimura, N.; Wong, M. C.; Hardisty, G.; Higgins, B.; Huang, P.-C.; Besh, M.; Kendall, T. J.; Rossi, A.; Ramachandran, P.; Patten, D.; Shetty, S.; Anderson, G.; Withers, D.; Tsuchiya, A.; Terai, S.; Lu, W.-Y.
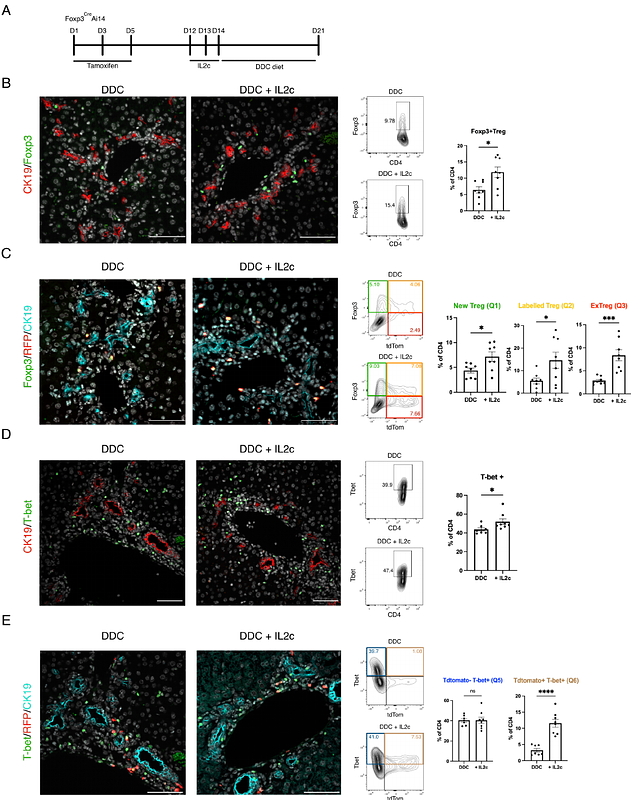
AbstractCholangiopathies such as Primary Sclerosing Cholangitis (PSC) cause damage to the bile ducts and fibrosis with no effective cure. A reduction and impairment in the function of regulatory T cells (Tregs) occurs in PSC. Yet, it is currently unknown what consequence this has on bile duct regeneration. We investigate whether Tregs regulate bile duct regeneration and the dynamics of Tregs turnover during bile duct injury. We used the transgenic Foxp3GFPDTR model to mimic reduced Tregs infiltration to the liver during bile duct injury and showed that reduced intrahepatic Tregs limits bile duct regeneration. Fate mapping of Tregs (Foxp3CreERTAi14) showed that Tregs acquire a pro-inflammatory phenotype within the inflammatory microenvironment, even after IL2 mediated Tregs expansion. Ox40L expression correlates with fibrosis in PSC patients. In the 3,5-Diethoxycarbonyl-1,4-Dihydrocollidine (DDC)-diet mouse model of experimental cholangiopathy, combining IL2-complex administration and blocking Ox40L decreases periportal fibrosis level, increases Treg number and reduces the pro-inflammatory phenotype of Tregs. These results indicate that Tregs mediate cholangiocyte response to biliary injury, and Tregs downregulate Foxp3 and acquire an inflammatory phenotype in an inflammatory microenvironment. Enhancing Treg numbers through IL2 complex administration and blocking Ox40 signalling simultaneously suppress the inflammatory phenotype of Tregs and reduces bile duct damage and fibrosis.