Scaling of mouse somitogenesis by coupling of cell cycle to segmentation clock oscillations
Scaling of mouse somitogenesis by coupling of cell cycle to segmentation clock oscillations
van Oostrom, M. v.; Li, Y. I.; Meijer, W. H. M.; Noordzij, T. E. J. C.; Fountas, C.; Timmers, E.; Korving, J.; Thomas, W. M.; Simons, B. D.; Sonnen, I.
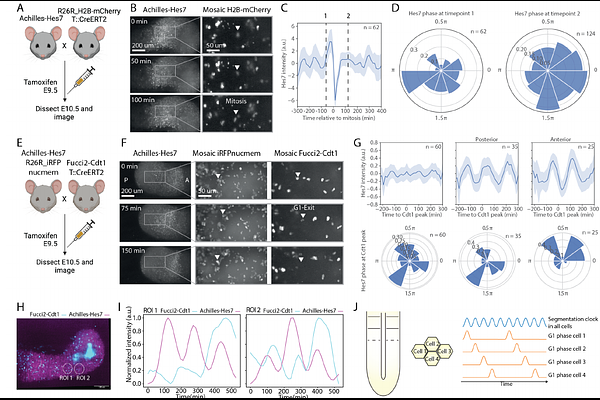
AbstractSomitogenesis is the periodic segmentation of a continuously growing tissue in vertebrate embryos. Timing and spacing of somitogenesis is regulated by the segmentation clock, oscillations in Notch, Wnt and FGF signalling in mice, and FGF and Wnt morphogen gradients. The size of nascent somites scales with the size of the unsegmented region, the presomitic mesoderm (PSM), over developmental time and upon experimental perturbation of PSM size. Here, we addressed how scaling is ensured in the developing mouse embryo. We found that the entire oscillating presomitic mesoderm proliferates. Quantifying the dynamics of signalling and cell cycle progression in single cells, we detected a correlation between the two. Using a microfluidics-based entrainment approach, we showed that signalling oscillations and cell cycle are coupled, likely via periodic induction of S phase-inducing Cyclins. An updated theoretical somitogenesis model incorporating this coupling suggests that this correlation ensures uniform PSM growth and uniform dilution of morphogen gradients by cell divisions, thereby directly affecting somite formation. Indeed, when blocking cell proliferation, gradient spreading and somite formation as well as somite scaling are impaired. Our data suggests a mechanism by which coupling signalling oscillations to cell proliferation ensures formation of properly sized somites and scaling of somite size.