Construction of Animal Models Based on Exploring Pathological Features and Mechanisms of Different Locations in the Progression of DVT-APTE-CTEPD/CTEPH
Construction of Animal Models Based on Exploring Pathological Features and Mechanisms of Different Locations in the Progression of DVT-APTE-CTEPD/CTEPH
Huang, L. Q.; Feng, W. W.; Yun, C. X.; Xiang, L. J.; Nan, S.; Xia, W. Q.; Yue, L. X.; He, C. M.; Min, C.; Jing, W. Y.; Wen, W. D.; Li, L. H.; Ran, Y. P.; Xia, Z. Y.; Zhu, Z.; Guo, Z. Z.; Sheng, D. C.
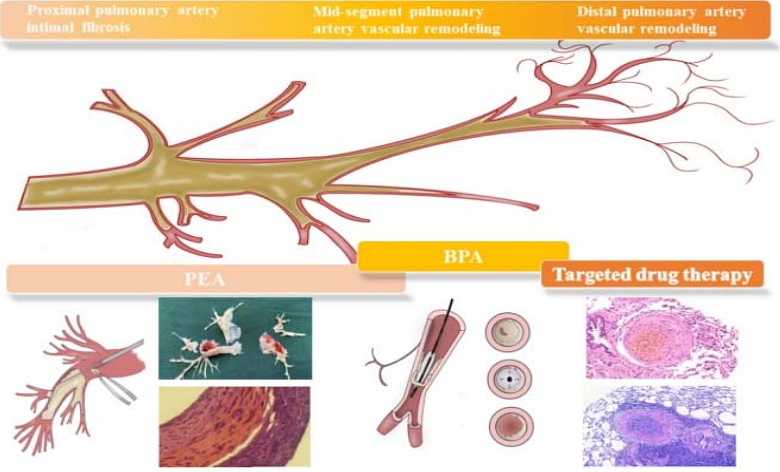
AbstractBackground: Chronic thromboembolic pulmonary disease (CTEPD) and chronic thromboembolic pulmonary hypertension (CTEPH) are sequelae of acute pulmonary embolism (APE) and severely affect patients\' health and quality of life. The treatment of these conditions is challenging, and their underlying mechanisms remain unclear. The main reason for this is the lack of an animal model that can fully simulate the entire chain of DVT-APTE-CTEPD/CTEPH progression. The objective of this study is to construct an ideal animal model that simulates the major pathological changes of DVT-APTE-CTEPD/CTEPH and can be used for mechanistic exploration. We aim to compare the advantages and disadvantages of different modeling approaches and provide an experimental basis for investigating the mechanisms of pulmonary embolism chronicization at different stages of evolution. Methods and Materials: We first evaluated the pathological changes in the pulmonary arterial intima stripping tissue of CTEPH patients. Animal models were established by multiple injections of thrombus columns through the internal jugular vein to simulate distal remodeling of the pulmonary artery. To simulate significant remodeling and fibrosis in the middle and distal segments of the pulmonary artery, thrombus columns were injected along with splenectomy. A CTEPD model with intimal fibrosis remodeling was successfully established by selectively injecting large thromboemboli into the pulmonary artery sites in large animals (dogs). A rat model with pathological manifestations of intimal fibrosis remodeling in the proximal end of the pulmonary artery was constructed using large thrombi combined with nitric oxide synthase inhibitors. An animal model of DVT was established using the inferior vena cava ligation method. Results: According to the different pathological features and mechanisms observed in the progression of human DVT-APTE-CTEPD/CTEPH, we constructed animal models that conform to these pathological manifestations and mechanisms, each with its own advantages. Furthermore, the different methods used to construct animal models can be integrated and applied together. Conclusion: Animal models constructed using different modeling methods can effectively simulate the pathological and physiological manifestations of the corresponding stages of chronic pulmonary embolism. Researchers can select the aforementioned models according to their specific research purposes, directions, and requirements.


