C-terminal tagging, transmembrane domain hydrophobicity, and an ER retention motif influence the secretory trafficking of the inner nuclear membrane protein emerin
C-terminal tagging, transmembrane domain hydrophobicity, and an ER retention motif influence the secretory trafficking of the inner nuclear membrane protein emerin
Mella, J.; Volk, R. F.; Zaro, B. W.; Buchwalter, A.
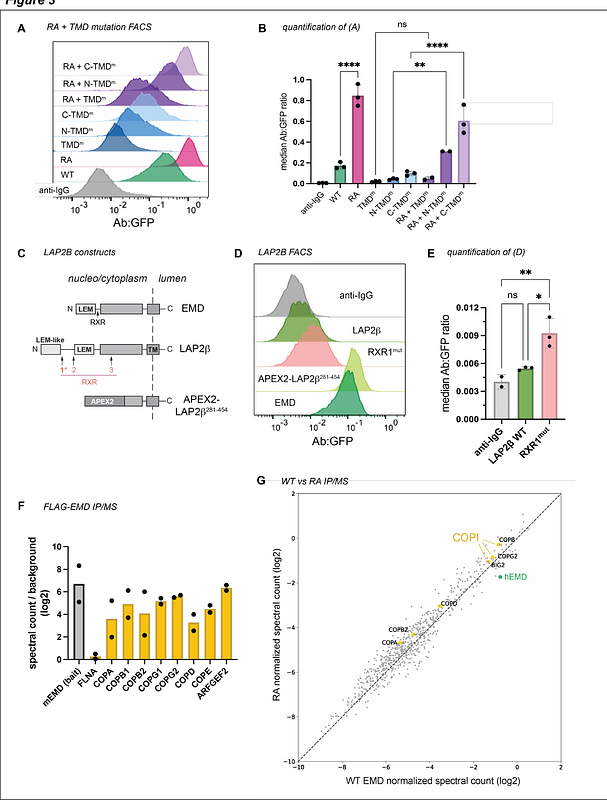
AbstractThe inner nuclear membrane (INM), a subdomain of the endoplasmic reticulum (ER), sequesters hundreds of transmembrane proteins within the nucleus. We previously found that one INM protein, emerin, can evade the INM by secretory transport to the lysosome, where it is degraded. In this work, we used targeted mutagenesis to identify intrinsic sequences that promote or inhibit emerins secretory trafficking. By manipulating these sequences across several tag and expression level combinations, we now find that emerins localization is sensitive to C-terminal GFP tagging. While emerins long, hydrophobic C-terminal transmembrane domain facilitates trafficking to the lysosome, extending its lumenal terminus with a GFP tag biases the protein toward this pathway. In contrast, we identify a conserved ER retention sequence that stabilizes N- and C-terminally tagged emerin by limiting its lysosomal flux. These findings underscore long-standing concerns about tagging artifacts and reveal novel determinants of tail-anchored INM protein targeting.