Mechanism and energetics of JDP induced Hsp70's conformational transition towards catalytically active state
Mechanism and energetics of JDP induced Hsp70's conformational transition towards catalytically active state
Olewniczak, M.; Pitek, M.; Czub, J.; Marszalek, J.; Nierzwicki, L.; Tomiczek, B.
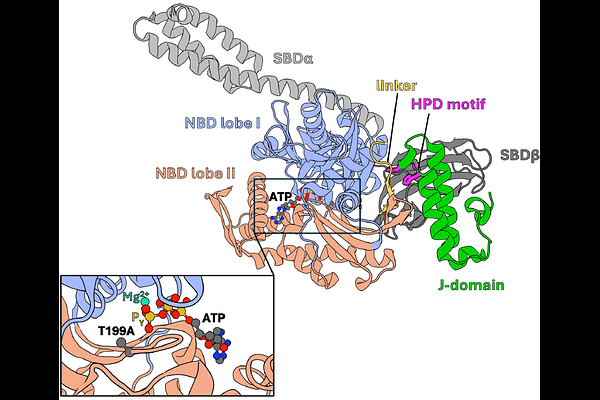
AbstractHsp70 chaperones are crucial for maintaining protein homeostasis by regulating the stability and conformational states of client polypeptides through cycles of their binding and release. These cycles require conformational transitions of Hsp70 driven by ATP binding and hydrolysis. The ATPase activity of Hsp70 is controlled by J-domain protein (JDP) cochaperones, which allosterically stimulate ATP hydrolysis via interactions between their J-domains and Hsp70. The J-domain binds at the interface between the nucleotide (NBD) and substrate (SBD) binding domains of ATP bound Hsp70. Although, it was established that the JD interaction involves residues of helices II and III, and the interhelical loop critical for ATPase stimulation, the mechanism by which the allosteric signal induced by J-domain binding is transmitted to the distal nucleotide-binding pocket of Hsp70 remains unclear, as do the conformational changes leading to the ATP hydrolysis. Here, we addressed these questions by means of all-atom free energy simulations and dynamic network analysis, starting from the crystal structures of ATP-bound Hsp70 DnaK alone and in complex with the J-domain of DnaJ. We demonstrated that the presence of the J-domain results in the rearrangement of the nucleotide-binding pocket into a hydrolysis competent state, characterized by close contact between universally conserved T199 of NBD and {gamma}-phosphate of ATP. With network analysis we revealed that the allosteric signal for this rearrangement is transmitted along the {beta}-strand containing T199. Finally, we provide rationale for the signal transmission, where steric repulsion between the J-domain\'s helix III and SBD induces a push of the T199 containing {beta}-strand. Overall, our study provides mechanistic insights into allosteric signal transmission within Hsp70, bridging the gap between J-domain binding and ATPase stimulation.