Mechanisms of force transmission on cytokinesis Formin Cdc12 in fission yeast revealed by coiled-coil force sensors
Mechanisms of force transmission on cytokinesis Formin Cdc12 in fission yeast revealed by coiled-coil force sensors
Saito, T.; Ren, Y.; Berro, J.
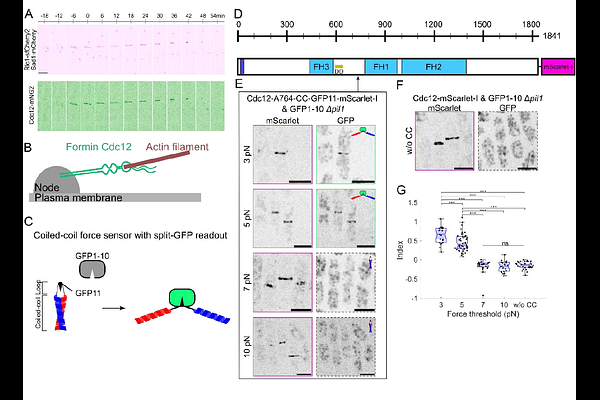
AbstractCytokinesis is a fundamental process in cell division, where an actomyosin contractile ring plays a central role in completing the cell division. Although some experimental and computational efforts have evaluated ring tension and the molecular organization of rings, the mechanisms of force transmission at the molecular level remain unclear. Here, we used our novel coiled-coil force sensors to measure the force distribution along the formin Cdc12, a key cytokinesis protein in fission yeast. Our force measurements revealed that individual formin Cdc12 molecules transmit up to ~6 pN with distinct mechanisms between the regions upstream and downstream of the formin homology 2 (FH2) domain, which binds the barbed ends of actin filaments. The force transmitted on the N-terminal region before the FH2 domain requires anchoring to a cytokinetic node via the Cdc15 binding region, but is independent of the C-terminal region after FH2. In contrast, the region after the FH2 domain transmits forces independently of the Cdc12 N-terminal region. Force screening using the coiled-coil force sensors found that a region in Cdc12 disordered C-terminal tail can associate with the contractile ring. Altogether, the force measurements with our coiled-coil force sensors allowed us to uncover the mechanisms of force transmission along Cdc12 and characterize new motifs and binding partners.