Computational demonstration of spinal circuit that modulates γ-MN activity via α-MN collateral mitigates the inevitable disruptions from velocity-dependent stretch reflexes during voluntary movements
Computational demonstration of spinal circuit that modulates γ-MN activity via α-MN collateral mitigates the inevitable disruptions from velocity-dependent stretch reflexes during voluntary movements
Niyo, G.; Almofeez, L. I.; Erwin, A.; Valero-Cuevas, F. J.
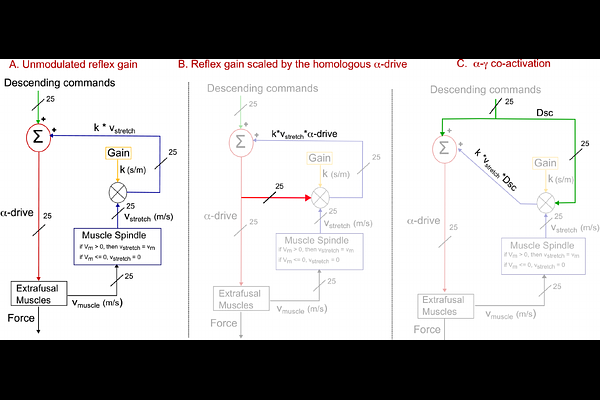
AbstractThe primary motor cortex does not uniquely or directly produce -MN drive to muscles during voluntary movement. Rather, -MN drive emerges from the synthesis and competition among excitatory and inhibitory inputs from multiple descending tracts, spinal interneurons, sensory inputs, and proprioceptive afferents. One such fundamental input are velocity-dependent stretch reflexes in lengthening (antagonist) muscles, which the shortening (agonist) muscles are thought to inhibit to allow voluntary movement. It remains an open question, however, the extent to which velocity-dependent stretch reflexes disrupt voluntary movement, and whether and how they should be inhibited in limbs with numerous mono- and multi-articular muscles where agonist and antagonist roles become unclear and can switch during a movement. We address these long-standing fundamental questions using 3D movements against gravity in a 25-muscle computational model of a Rhesus Macaque arm. After simulating 1,100 distinct movements across the workspace of the arm with feedforward -MN commands, we computed the kinematic disruptions to the arm endpoint trajectories caused by adding positive homonymous muscle velocity feedback (i.e., simple velocity-dependent stretch reflexes ) at different static gains to the feedforward -MN drive (without reciprocal inhibition). We found that arm endpoint trajectories were disrupted in surprisingly movement-specific, typically large and variable ways, and could even change movement direction as the reflex gain increased. In contrast, these disruptions became small at all reflex gains when the velocity-dependent stretch reflexes were simply scaled by the -MN drive to each muscle (equivalent to an -MN excitatory collateral to its homologous {gamma}-MNs , but distinct from -{gamma} co-activation ). We argue this circuitry is more neuroanatomically tenable, generalizable, and scalable than -{gamma} co-activation and movement-specific reciprocal inhibition. In fact, we propose that this mechanism at the homonymous propriospinal level could be a critical low-level enabler of learning via cerebellar and cortical mechanisms by locally and automatically regulating the highly nonlinear neuro-musculo-skeletal mechanics of the limb. This propriospinal mechanism also provides a powerful paradigm that may begin to clarify how dysregulation of {gamma}-MN drive can result in disruptions of voluntary movement in neurological conditions.