Diffusion MRS tracks distinct trajectories of neuronal development in the cerebellum and thalamus of rat neonates.
Diffusion MRS tracks distinct trajectories of neuronal development in the cerebellum and thalamus of rat neonates.
Ligneul, C.; Qiu, L.; Clarke, W. T.; Jbabdi, S.; Palombo, M.; Lerch, J.
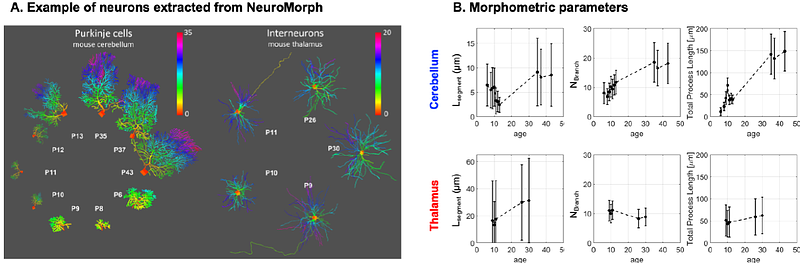
AbstractThe brain develops in a nonuniform manner, with local bursts of plasticity. In mammals, the cerebellum remains in a vulnerable developmental state longer than the neocortex, making it a potential hotspot for neurodevelopmental disorders. Cerebellar neurons, and more particularly Purkinje cells, have an altered morphology in forms of autism spectrum disorders or in Down syndrome. However, it is currently impossible to non-invasively assess cerebellar cell-structure during early development. Here we use diffusion-weighted magnetic resonance spectroscopy in combination with microstructural modelling to longitudinally track cerebellar cell-specific development from P5 to P30 in rat neonates. Tracking metabolite diffusion allows us to probe cell-specific developmental trajectories. Additionally, by comparing different analytical and biophysical microstructural models we can follow the differential contribution of cell bodies and neurites during development. The thalamus served as a control region to assess the sensitivity of our method to microstructural differences between the regions. We found significant differences between cerebellar and thalamic metabolites diffusion properties. We report a decreased sphere fraction (or an increased neurite fraction) with age for taurine and total creatine in the cerebellum, marking dendritic growth. Surprisingly, we also report a U-shape trend for segment length (the distance between two embranchments in a dendritic tree) in the cerebellum with age matching morphometry of openly available 3D-Purkinje reconstructions. Results demonstrate that diffusion-weighted MRS probes early cerebellar neuronal development non-invasively.