EGFR-Mediated Mechanotransduction in Aortic Valve Cells: A Key Pathway in Response to Wall Shear Stress
EGFR-Mediated Mechanotransduction in Aortic Valve Cells: A Key Pathway in Response to Wall Shear Stress
Gaste, A.; Marchese, D.; Faucherre, A.; Lopez, L.; Bertrand, E.; THERON, A.; Herbane, M.; Seddik, A.; Jopling, C.; Avierinos, J.-F.; Ait Oufella, H.; Deplano, V.; Zaffran, S.
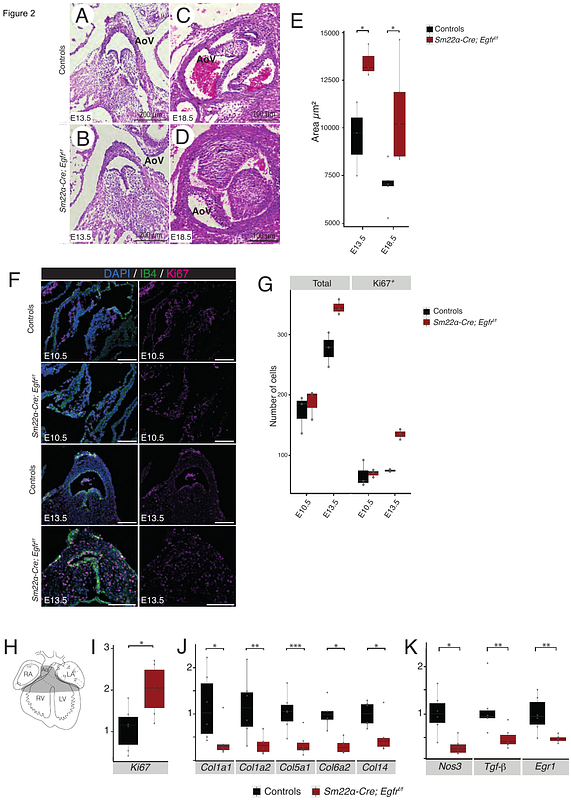
AbstractAim: Blood flow-induced mechanical forces, particularly wall shear stress (WSS), play a fundamental role in aortic valve remodeling and maturation. Dysregulation of these processes contributes to age-related valve diseases, such as aortic stenosis and regurgitation. While epidermal growth factor receptor (EGFR) signaling has been implicated in valve development, its role in mechanotransduction remains unclear. This study aims to investigate how EGFR regulates WSS-induced signaling in valvular cells and explore its interaction with the mechanosensitive ion channel PIEZO1. Methods and Results: To investigate the role of EGFR in valvular cell mechanotransduction, we used conditional Egfrflox allele to selectively delete Egfr in valvular cells. Histological analysis revealed increased valve leaflet thickness and hyperproliferation of mesenchymal cells when Egfr was deleted both endothelial (Tie2-Cre lineage) and mesenchymal (Sm22 alpha-Cre lineage) cells. This was accompanied by a reduction in maturation-related genes (Egr1, Nos3, Tgf-beta) and extracellular matrix (ECM) components. We previously demonstrated that Egr1 expression is regulated by WSS in valvular endothelial cells, prompting further exploration of Egr1\'s role in valvular cells. In vitro, Egr1 overexpression and shRNA-mediated knockdown confirmed its role in regulating Nos3, Col1a1, and Tgf-beta, key mediators of valve remodeling. Using a pulsatile WSS-mimicking device, we found that WSS induces Erk1/2 phosphorylation and Egr1 expression in valvular cells, both of which were abolished by EGFR inhibition. However, direct EGFR activation via EGF failed to replicate WSS-induced Egr1 expression, suggesting the involvement of additional mechanosensitive pathways. Pharmacological studies further revealed that PIEZO1 inhibition impaired WSS-induced Egr1 expression, while PIEZO1 activation (via YODA) mimicked WSS effects on Erk1/2 phosphorylation and Egr1 expression. These findings suggest a functional interaction between EGFR and PIEZO1 in mechanotransduction, linking mechanical forces to key molecular pathways in valve remodeling. Conclusion: Our findings establish EGFR as a critical mediator of WSS-induced mechanotransduction in valve remodeling, working in synergy with PIEZO1 to regulate flow-sensitive transcription factors such as Egr1. This study provides new insights into the molecular mechanisms governing valve maturation and highlights potential therapeutic targets for age-related valve pathologies linked to abnormal WSS responses.