Chloroplasts lacking class I glutaredoxins are functional but show a delayed recovery of protein cysteinyl redox state after oxidative challenge
Chloroplasts lacking class I glutaredoxins are functional but show a delayed recovery of protein cysteinyl redox state after oxidative challenge
Bohle, F.; Rossi, J.; Tamanna, S. S.; Jansohn, H.; Schlosser, M.; Reinhardt, F.; Brox, A.; Bethmann, S.; Kopriva, S.; Trentmann, O.; Jahns, P.; Deponte, M.; Schwarzländer, M.; Trost, P.; Zaffagnini, M.; Meyer, A. J.; Müller-Schüssele, S. J.
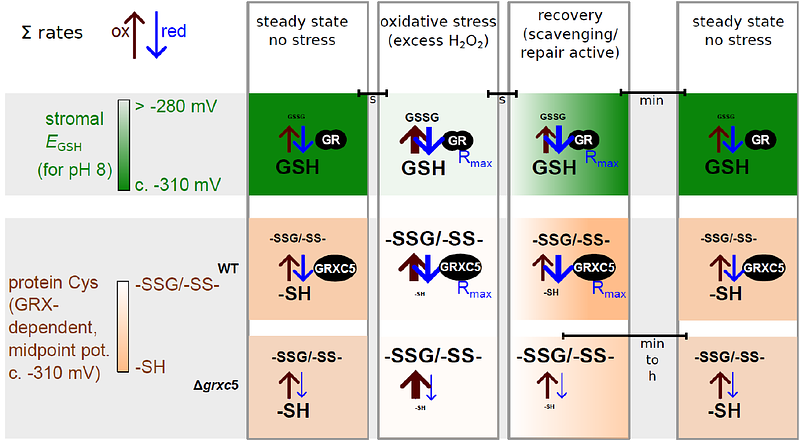
AbstractRedox status of protein cysteinyl residues is mediated via glutathione (GSH) /glutaredoxin (GRX) and thioredoxin (TRX)-dependent redox cascades. An oxidative challenge can induce post-translational protein modifications on thiols, such as protein S-glutathionylation. Class I GRX are small thiol-disulfide oxidoreductases that reversibly catalyse S-glutathionylation and protein disulfide formation. TRX and GSH/GRX redox systems can provide partial backup for each other in several subcellular compartments, but not in the plastid stroma where TRX/light-dependent redox regulation of primary metabolism takes place. While the stromal TRX system has been studied at detail, the role of class I GRX on plastid redox processes in vivo is still unknown. We generate knockout lines of GRXC5 as the only chloroplast class I GRX of the moss Physcomitrium patens. While we find that class I PpGRXC5 has high activities in glutathione-dependent oxidoreductase assays using hydroxyethyl disulfide or redox-sensitive GFP2 (roGFP2) as substrates in vitro, {Delta}grxc5 plants show no detectable growth defect or stress sensitivity, in contrast to mutants with a less negative stromal EGSH ({Delta}gr1). Using stroma-targeted roGFP2, we show increased protein Cys oxidation and decreased reduction rates after oxidative challenge in {Delta}grxc5 plants in vivo, indicating kinetic uncoupling of the protein Cys redox state from glutathione redox potential. Protein Cys disulfide and S-glutathionylation formation rates after H2O2 treatment remained unchanged. Lack of class I GRX function in the stroma did not result in impaired carbon fixation. Our observations suggest specific roles for class I GRX in the efficient redox equilibration between EGSH and protein Cys in the plastid stroma as well as negligible cross-talk with metabolic regulation via the TRX system. We propose a model for stromal class I GRX function as efficient kinetic couplers of protein Cys redox state to the dynamic stromal EGSH and highlight the importance of identifying in vivo target proteins of GRXC5.