Repressive S-adenosylmethionine biosynthesis status inhibits transcription of HeT-A retrotransposon in the germline of Drosophila.
Repressive S-adenosylmethionine biosynthesis status inhibits transcription of HeT-A retrotransposon in the germline of Drosophila.
Hayashi, Y.; Hino, S.; Sato, T.; Kashio, S.; Saito, K.; Sato, B.; Kawano, N.; Saito, D.; Miura, M.; Suyama, M.; Nakao, M.; Kobayashi, S.
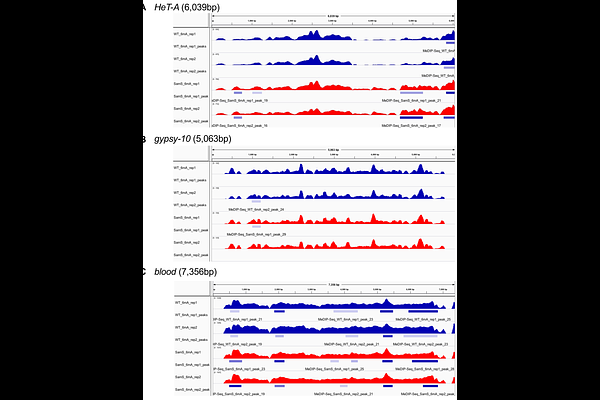
AbstractS-adenosylmethionine (SAM) is the principal cellular donor of methyl moiety in the methylation reaction and regulates gene expression by regulating methylation-related cellular events, such as epigenetic status. Although SAM biosynthesis affects a variety of biological phenomena including disease and aging, whether cell-specific SAM biosynthesis status is present and how it contributes to cellular function are largely unknown. Here, we firstly showed that the Drosophila germline in gametogenesis has a repressive SAM biosynthesis status through the observation of SAM synthetase (Sam-S), a key enzyme for SAM biosynthesis. In addition, our study showed that germline-unique repressive SAM biosynthesis status contributes to inhibition of retrotransposon expression; enhancement of SAM biosynthesis in germline caused excessive expression of retrotransposons including HeT-A, a telomere-specific retroelement, as the most affected target. We found that the promoter activity of HeT-A is enhanced in SAM increased condition with increased accumulation of 6mA DNA methylation, the major DNA methylation modification in the Drosophila genome. Interestingly, the enhanced 6mA enrichment and gene expression in enriched loci were not correlated in other retrotransposons or structural genes. Taken together, our results suggest that SAM-deficient status in the germline uniquely regulates HeT-A transcription via 6mA methylation modification. Thus, our study provides a new understanding of how germline unique metabolic status contributes to regulation of the retrotransposon.