Intravenous BCG immunization drives naive CD4+ T cell to Th1 differentiation via CD4+ T cell epigenetic reprogramming
Intravenous BCG immunization drives naive CD4+ T cell to Th1 differentiation via CD4+ T cell epigenetic reprogramming
Wang, R.; Zhao, Y.; Yang, J.; Luo, R.; Sun, W.; Zhang, M.; Xiang, D.; Hou, Y.; Cao, P.; Li, E.
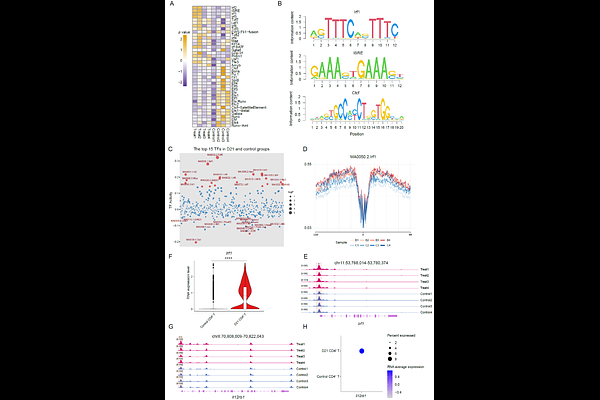
AbstractIntravenous (IV) administration of live Bacille Calmette-Guerin (BCG) boosts potent immunity through coordinated interactions of innate immune response and adaptive immune response for BCG antiviral activity. The underlying mechanisms by which BCG initiates CD4+ T response remain incompletely understood. Here, we show that IV administration of BCG drives naive CD4+ T cell differentiation to Th1 cell through epigenetic reprogramming. Single-cell transcriptomic and epigenomic analyses revealed that IV BCG immunization induced metabolic reprogramming in monocytes and increased the proportion of T cells. The immunization induced chromatin openness for transcription factors regulating naive CD4+ T cells differentiation into Th1 cells. Importantly, we observed increases in Stat1, Irf1 and interferon-stimulated genes such as Igtp in Th1 cells, leading to upregulation of interferon related gene expression for antiviral response by BCG immunization. Activated transcription factors like Irf1 in CD4+ T cells promotes Il12rb1 expression to facilitate IL-12 signaling and drive naive CD4+ T cells differentiation into Th1 cells. We showed that BCG immunization reduced respiratory syncytial virus (RSV) infection. Thus, transcription regulation through epigenetic reprogramming plays a critical role in BCG-induced CD4+ T cell differentiation and to the broad antiviral activity.