The mycobacterial glycoside hydrolase LamH enables capsular arabinomannan release and stimulates growth
The mycobacterial glycoside hydrolase LamH enables capsular arabinomannan release and stimulates growth
Franklin, A.; Layton, A. J.; Mize, T.; Salgueiro, V. C.; Sullivan, R.; Benedict, S. T.; Gurcha, S. S.; Anso, I.; Besra, G. S.; Banzhaf, M.; Lovering, A. L.; Williams, S. J.; Guerin, M. E.; Scott, N. E.; Prados-Rosales, R.; Lowe, E. C.; Moynihan, P. J.
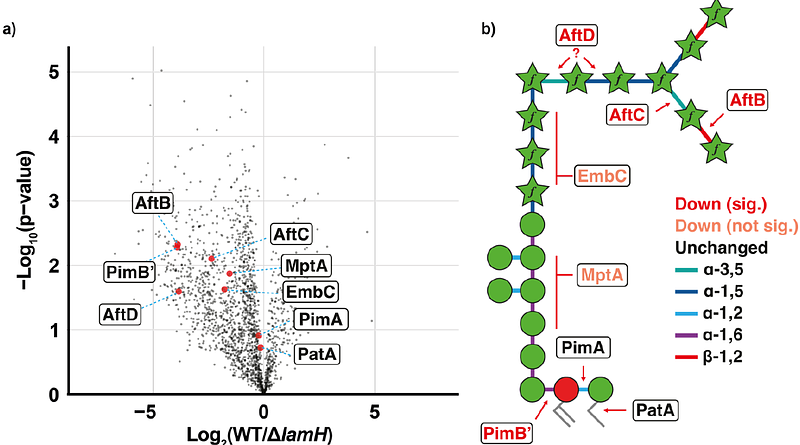
AbstractMycobacterial glycolipids are important cell envelope structures that drive host-pathogen interactions. Arguably, the most important amongst these are lipoarabinomannan (LAM) and its precursor, lipomannan (LM); both are trafficked out of the bacterium to the host via completely unknown mechanisms. An important class of exported LM/LAM is the capsular derivative of these molecules which is devoid of its lipid anchor. Here, we describe the identification of LamH, a glycoside hydrolase (GH) family 76 enzyme that specifically cleaves LM/LAM, driving its export to the capsule releasing its phosphatidyl-myo-inositol mannoside lipid anchor. Unexpectedly, we found that the catalytic activity of this enzyme is important for efficient exit from stationary phase cultures where arabinomannan acts as a signal for growth phase transition. Finally, we demonstrate that LamH is important for Mycobacterium tuberculosis survival in macrophages. These data provide a new framework for understanding the biological role of LAM in mycobacteria.