Transient lung eosinophilia during breakthrough influenza infection in vaccinated mice is associated with protective and balanced Type 1/2 immune responses.
Transient lung eosinophilia during breakthrough influenza infection in vaccinated mice is associated with protective and balanced Type 1/2 immune responses.
Chang, L. A.; Yeung, S. T.; Warang, P.; Noureddine, M.; Singh, G.; Webb, B. T.; Schotsaert, M.
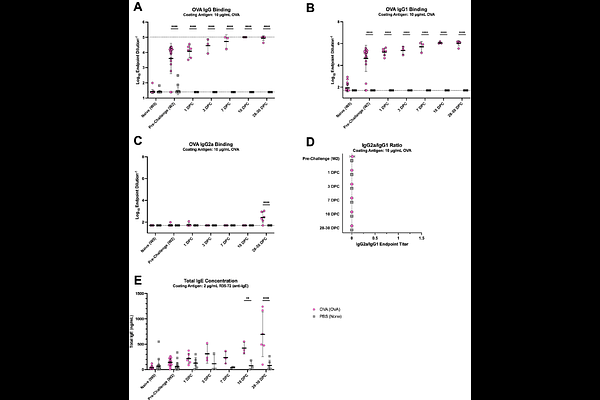
AbstractEosinophils are agile cells that participate in a multitude of homeostatic and inflammatory responses in the lung, ranging from allergic asthma to antiviral defense against respiratory viral infection. In the context of vaccination followed by viral infection, such as breakthrough infection, eosinophils have been linked to aberrant Th2 responses like vaccine-enhanced respiratory disease (VAERD). Here, we demonstrate that the lung immune cell composition, cytokine and chemokine repertoire, histopathological profile, and systemic humoral response of breakthrough influenza infection in mice is distinct from that of primary influenza infection or allergic sensitization, canonical Type 1 and 2 immune responses, respectively. Longitudinal comparison of breakthrough infection with allergic sensitization and primary influenza infection demonstrated major differences in lung immunity between treatment groups in female, BALB/c mice. Breakthrough infection mice exhibit lung eosinophil infiltration that peaks at 7-10 days post-challenge, enriched for the Siglec-Fhi subset, but in the absence of overt pro-inflammatory cytokine/chemokine signals, high viral titers, severe lung lesions, goblet cell hyperplasia, allergic levels of total IgE, or enhanced morbidity. Multiparameter fluorescence imaging corroborated findings from flow cytometry and also unveiled interactions between CD101+Siglec-F+ cells with CD3+ cells in the lung tissue space. Imaging also revealed a marked absence of eosinophil or neutrophil extracellular traps in the lungs of breakthrough infection mice, in contrast with allergic sensitization and primary influenza infection, respectively. Altogether, our findings provide a deeper understanding of the kinetics and cell-cell interplay during non-pathological, balanced Type 1/2 immune responses in vaccinated hosts during breakthrough infection.