High-Throughput Centrifuge Force Microscopy Reveals Dynamic Immune-Cell Avidity at the Single-Cell Level
High-Throughput Centrifuge Force Microscopy Reveals Dynamic Immune-Cell Avidity at the Single-Cell Level
Bergal, H. T.; Kinoshita, K.; Wong, W. P.
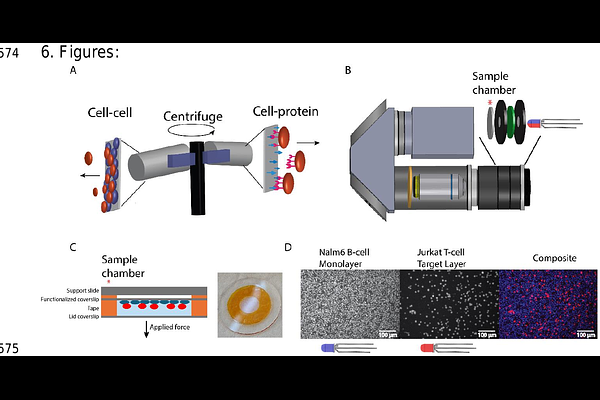
AbstractCell-cell binding, mediated by the physical interactions of receptors and their ligands, plays a fundamental role in immune processes such as immune surveillance and T-cell activation. However, current approaches for measuring cell avidity often lack either throughput or quantitative precision. Here, we introduce a high-throughput approach for quantifying cell binding lifetimes and strength using a centrifuge force microscope (CFM)--a compact microscope operating within a standard benchtop centrifuge. The CFM enables live monitoring of single-cell interactions under force, conducting thousands of force experiments in parallel. To facilitate the real-time study of live cell interactions, we developed a next-generation CFM with multichannel fluorescence imaging capabilities. This system accommodates measurements in two modes: cell-protein binding and cell-cell avidity assays. Using this system, we investigated immune-cell binding mediated by Bispecific T-cell Engager (BiTE) molecules, a novel immunotherapy designed to enhance immune-cell targeting of cancer cells. In cell-protein assays, we quantified T- and B-cell unbinding from BiTE-functionalized surfaces, revealing receptor-specific relationships between ligand concentration and binding strength. In cell-cell assays, we examined BiTE-mediated binding of T-cells to Nalm6 B-cells, a precursor leukemia cell line, uncovering a strong, time-dependent increase in BiTE-mediated immune-cell avidity. By integrating high-throughput and quantitative single-cell force analysis, the CFM provides new insights into the dynamic nature of immunological interactions under force, with broad implications for immunotherapy and cellular mechanics.