Vaccine-elicited and naturally elicited antibodies differ in their recognition of the HIV-1 fusion peptide
Vaccine-elicited and naturally elicited antibodies differ in their recognition of the HIV-1 fusion peptide
Reveiz, M.; Xu, K.; Lee, M.; Wang, S.; Olia, A. S.; Harris, D. R.; Liu, K.; Liu, T.; Schaub, A. J.; Stephens, T.; Wang, Y.; Zhang, B.; Huang, R.; Tsybovsky, Y.; Kwong, P. D.; Rawi, R.
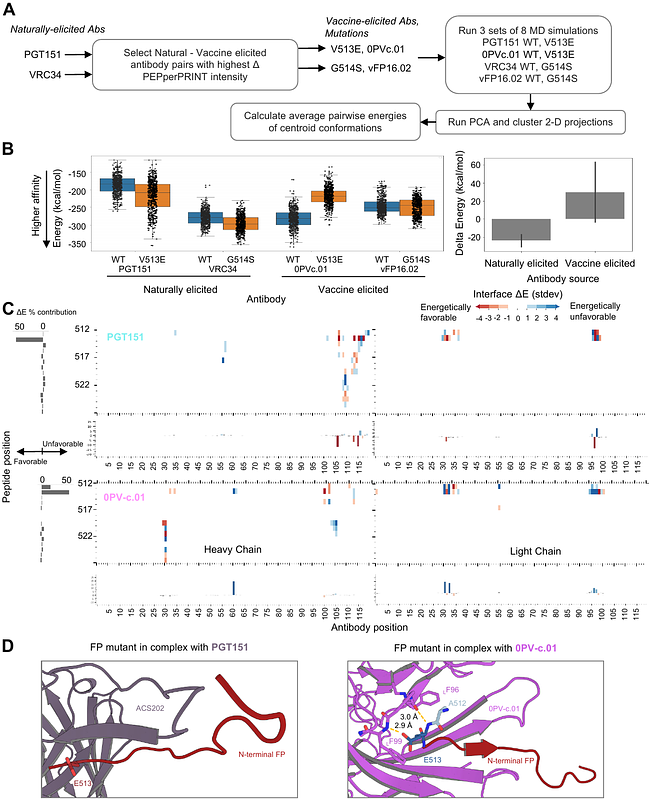
AbstractBroadly neutralizing antibodies have been proposed as templates for HIV-vaccine design, but it has been unclear how similar vaccine-elicited antibodies are to their naturally elicited templates. To provide insight, here we compare the recognition of naturally elicited and vaccine-elicited antibodies targeting the HIV-1-fusion peptide, which comprises envelope (Env) residues 512-526, with the most common sequence being AVGIGAVFLGFLGAA. Naturally elicited antibodies bound peptides with negative-charge substitutions around residues 517-520 substantially better than the most common sequence, despite these substitutions rarely appearing in HIV; by contrast, vaccine-elicited antibodies were less tolerant of sequence variation, with no substitution of residues 512-516 showing increased binding. Molecular dynamics analysis and cryo-EM structure of the naturally elicited ACS202 antibody in complex with HIV-Env trimer with A517E suggested enhanced binding to result from electrostatic interactions with positively charged antibody residues. Overall, vaccine-elicited antibodies appeared to be more fully optimized to bind the most common fusion peptide sequence.


