Sensory kinase KdpD is a tandem serine histidine kinase controlling K+ pump KdpFABC on the translational and post-transcriptional level
Sensory kinase KdpD is a tandem serine histidine kinase controlling K+ pump KdpFABC on the translational and post-transcriptional level
Silberberg, J. M.; Ketter, S.; Boehm, P. J.; Jordan, K.; Wittenberg, M.; Grass, J.; Haenelt, I.
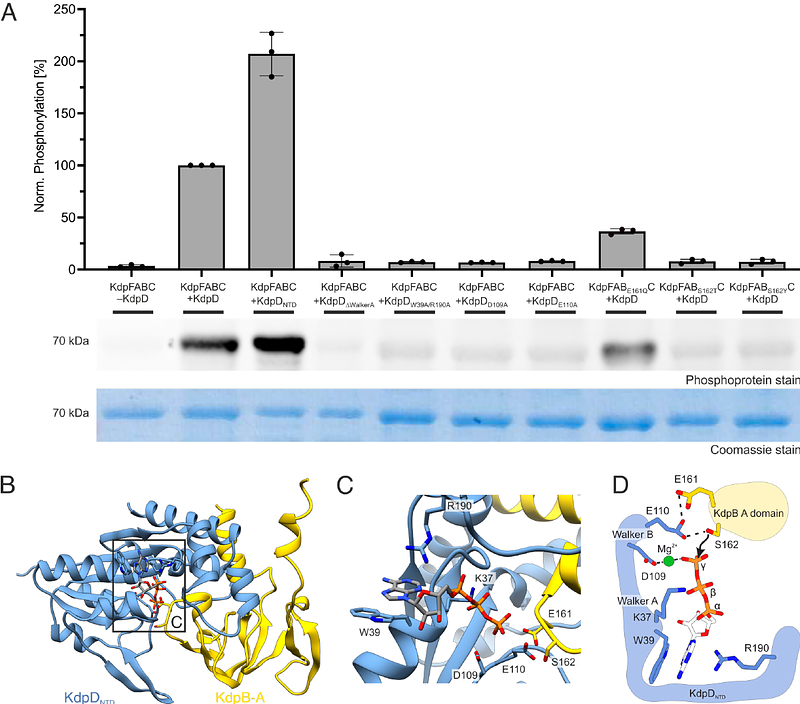
AbstractTwo-component systems (TCSs), consisting of a histidine kinase (HK) and a response regulator, serve signal transduction in bacteria, often regulating transcription in response to environmental stimuli. Here, we identify a tandem serine histidine kinase function for KdpD, previously described as a HK of the TCS KdpDE, which controls production of K+ pump KdpFABC. We show that KdpD additionally mediates an inhibitory serine phosphorylation of KdpFABC at high K+ levels, using not its C-terminal HK domain but an N-terminal atypical serine kinase (ASK) domain. Sequence analysis of KdpDs from different species highlights that some KdpDs comprise solely ASK and Usp domains. We show that, while Escherichia coli KdpD\'s ASK responds directly to K+ levels, a shorter version from Deinococcus geothermalis is controlled by second messenger cyclic di-AMP. Our findings add to the growing functional diversity of sensor kinases while simultaneously expanding the framework for regulatory mechanisms in bacterial K+ homeostasis.