1-Deoxysphingolipids dysregulate membrane properties and cargo trafficking in the early secretory pathway
1-Deoxysphingolipids dysregulate membrane properties and cargo trafficking in the early secretory pathway
Tsai, Y.-T.; Frederic-Lipp, N.; Varma, R.; Seidel, O.; Laguerre, A.; Solorio-Kirpichyan, K.; Wong, A.; Brea, R. J.; McGregor, G. H.; Cordes, T.; Devaraj, N. K.; Kuerschner, L.; Neal, S. E.; Metallo, C.; Budin, I.
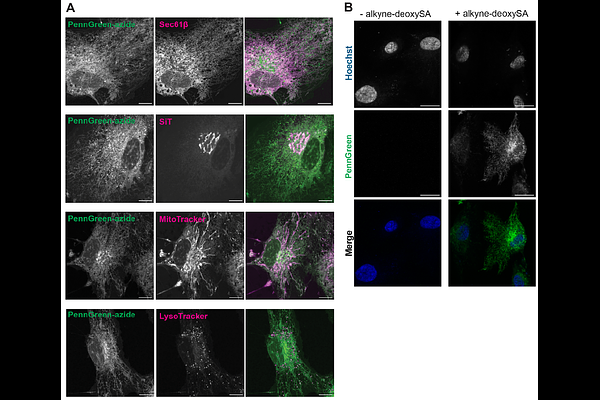
Abstract1-Deoxysphingolipids (1-deoxySL) are a non-canonical class of sphingolipids linked to genetic and metabolic disorders, including type II diabetes, hereditary sensory and autonomic neuropathy type 1, and macular telangiectasia (MacTel). In MacTel patients, retinal pigment epithelium (RPE) cells exhibit a loss of apical phagocytic receptors, suggesting a potential role for 1-deoxySLs in membrane trafficking. Here we use a range of lipid chemical biology approaches to investigate the effect of 1-deoxySLs on the properties and functions of secretory membranes. We first applied organelle-specific bioorthogonal labeling to visualize the subcellular distribution of metabolically-tagged 1-deoxySLs, observing that they become enriched in the ER of RPE-1 cells. While 1-deoxydihydroceramide is rapidly transported by the non-vesicular ceramide transporter CERT in vitro, they are retained in ER exit sites (ERES), suggesting that they do not efficiently sort into vesicular carriers leaving the ER. RPE-1 cells engineered to express disease-associated variants of serine palmitoyl transferase (SPT) were then used to test the consequences of this ER retention. Overproduction of 1-deoxySLs decreased ER membrane fluidity, as measured by the solvatochromic dye Laurdan, and enlarged ERES themselves. These effects could be partially phenocopied through inhibition of canonical sphingolipid metabolism and ceramide trafficking. Rates of synchronized protein cargo release from the ER were altered in response to 1-deoxySL accumulation in a manner that was dependent on cargo affinity for ordered or disordered membranes. We propose that altered lipid metabolism and trafficking can modulate the properties of secretory membranes, which could be relevant for protein trafficking in diseases with altered sphingolipid metabolism.