Computational design of monomeric Fc variants with distinct pH-responsive FcRn-binding profiles
Computational design of monomeric Fc variants with distinct pH-responsive FcRn-binding profiles
Jeon, J.-Y.; Lee, H.; Choi, S.; Suh, S.; Im, S.-B.; Kim, Y.-G.; Ku, B. M.; Ahn, M.-J.; Jeong, B.-s.; Oh, B.-H.
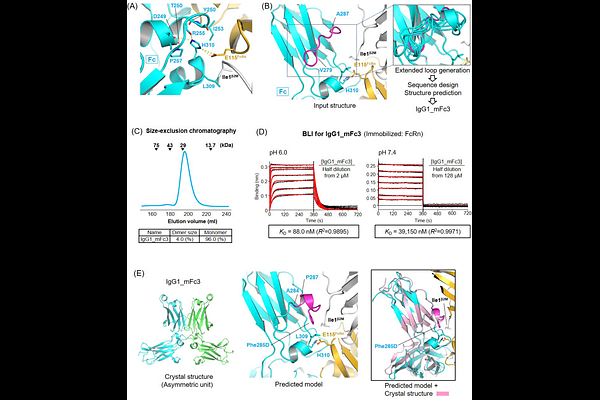
AbstractIgG1 and IgG4 antibodies form a [~]150 kDa homodimer through dimerization of the Fc domain, which prolongs their in vivo half-life via pH-dependent binding to the neonatal Fc receptor (FcRn). Conformationally stable, half-life-extended monomeric Fc (mFc) variants offer a promising platform for antibodies and Fc-fusion therapeutics, enabling deeper tissue penetration, reduced toxicity, and simplified manufacturing. Due to the loss of binding avidity, mFc requires significantly enhanced FcRn-binding affinity at pH 6.0, but retaining weak binding at neutral pH to achieve comparable serum half-life, making engineering such mFc variants highly challenging. Mainly by computational design approach, we created mFc mutants with diverse human FcRn-binding profiles, including two variants that exhibit 17- and 47-fold stronger FcRn binding at pH 6.0 (KD of 30.7 nM and 88 nM) compared to a baseline mFc, while maintaining greater than 213-fold weaker binding at pH 7.4 (KD of 6,549 nM and 39,150 nM). These variants are highly soluble and display a melting temperature greater than 60.6 {degrees}C, underscoring their potential as platforms for extending the in vivo half-life of therapeutic modalities. Other mFc variants with different pH-responsive FcRn-binding profiles would potentially fit for other therapeutic needs. Moreover, transferring the same variations into IgG4 Fc generated IgG4 mFc variants with FcRn-binding properties similar to those of the parent IgG1 mFc variants. Furthermore, incorporating the FcRn-binding affinity-enhancing substitutions into native Fc produced a dimeric Fc variant that exhibits strong, pH-responsive FcRn-binding affinities, promising an extended half-life in serum.