Ligand-Coupled Conformational Changes in a Cyclic Nucleotide-Gated Ion Channel Revealed by Time-Resolved Transition Metal Ion FRET
Ligand-Coupled Conformational Changes in a Cyclic Nucleotide-Gated Ion Channel Revealed by Time-Resolved Transition Metal Ion FRET
Eggan, P.; Gordon, S. E.; Zagotta, W. N.
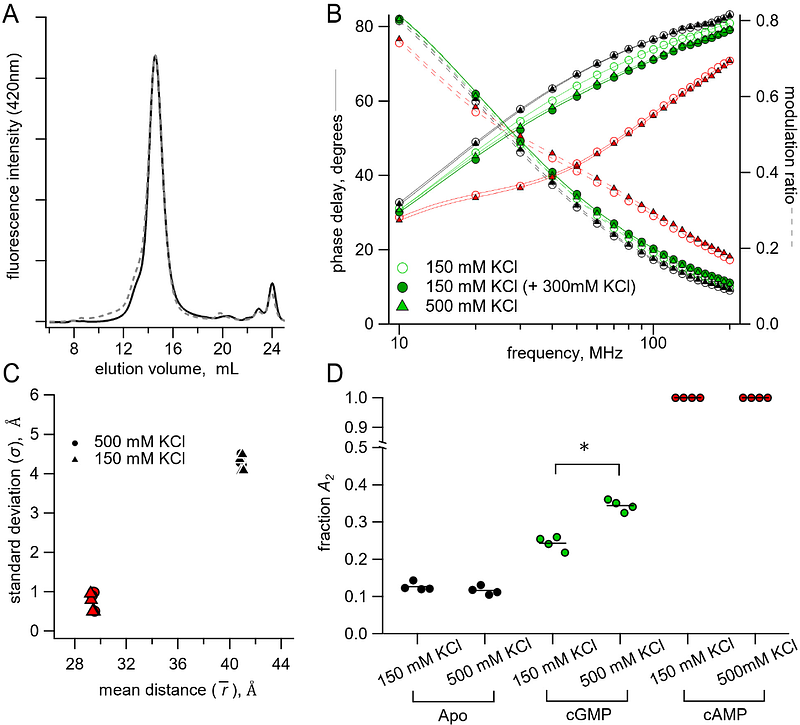
AbstractCyclic nucleotide-binding domain (CNBD) ion channels play crucial roles in cellular-signaling and excitability and are regulated by the direct binding of cyclic adenosine- or guanosine-monophosphate (cAMP, cGMP). However, the precise allosteric mechanism governing channel activation upon ligand binding, particularly the energetic changes within domains, remains poorly understood. The prokaryotic CNBD channel SthK offers a valuable model for investigating this allosteric mechanism. In this study, we investigated the conformational dynamics and energetics of the SthK C-terminal region using a combination of steady-state and time-resolved transition metal ion Forster resonance energy transfer (tmFRET) experiments. We engineered donor-acceptor pairs at specific sites within a SthK C-terminal fragment by incorporating a fluorescent noncanonical amino acid donor and metal ion acceptors. Measuring tmFRET with fluorescence lifetimes, we determined intramolecular distance distributions in the absence and presence of cAMP or cGMP. The probability distributions between conformational states without and with ligand were used to calculate the changes in free energy ({Delta}G) and differences in free energy change ({Delta}{Delta}G) in the context of a simple four-state model. Our findings reveal that cAMP binding produces large structural changes, with a very favorable {Delta}{Delta}G. In contrast to cAMP, cGMP behaved as a partial agonist and only weakly promoted the active state. Furthermore, we assessed the impact of protein oligomerization and ionic strength on the structure and energetics of the conformational states. This study demonstrates the effectiveness of time-resolved tmFRET in determining the conformational states and the ligand-dependent energetics of the SthK C-terminal region.
