NRF2-dependent regulation of the prostacyclin receptor PTGIR drives CD8 T cell exhaustion
NRF2-dependent regulation of the prostacyclin receptor PTGIR drives CD8 T cell exhaustion
Dahabieh, M. S.; DeCamp, L. M.; Oswald, B. M.; Kitchen-Goosen, S. M.; Fu, Z.; Vos, M.; Compton, S. E.; Longo, J.; Williams, K. S.; Ellis, A. E.; Johnson, A.; Sodiya, I.; Vincent, M.; Lee, H.; Sheldon, R. D.; Krawczyk, C. M.; Yao, C.; Wu, T.; Jones, R. G.
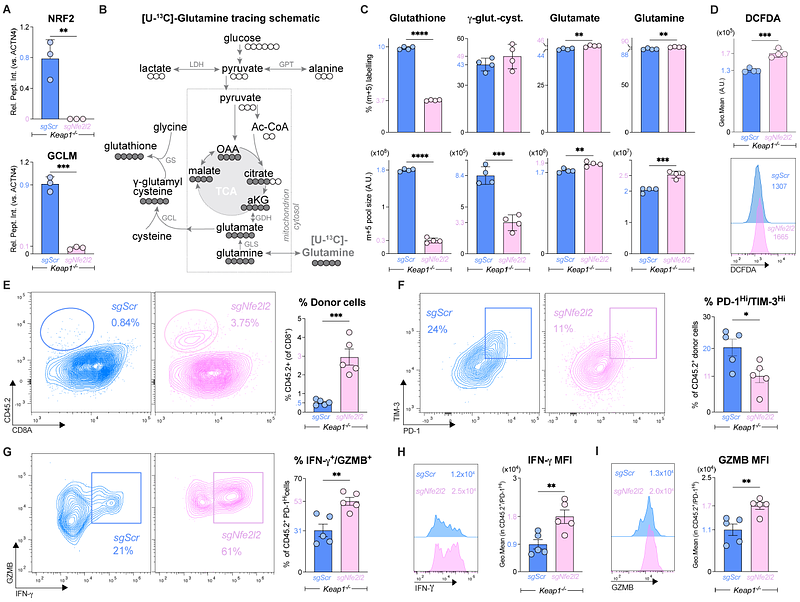
AbstractThe progressive decline of CD8 T cell effector function, also known as terminal exhaustion, is a major contributor to immune evasion in cancer. Yet, the molecular mechanisms that drive CD8 T cell dysfunction remain poorly understood. Here, we report that the Kelch-like ECH-associated protein 1 (KEAP1)-Nuclear factor erythroid 2-related factor 2 (NRF2) signaling axis, which mediates cellular adaptations to oxidative stress, directly regulates CD8 T cell exhaustion. Transcriptional profiling of dysfunctional CD8 T cells from chronic infection and cancer reveals enrichment of NRF2 activity in terminally exhausted (Tex-term) CD8 T cells. Increasing NRF2 activity in CD8 T cells (via conditional deletion of KEAP1) promotes increased glutathione production and antioxidant defense yet accelerates the development of terminally exhausted (PD-1+TIM-3+) CD8 T cells in response to chronic infection or tumor challenge. Mechanistically, we identify PTGIR, a receptor for the circulating eicosanoid prostacyclin, as an NRF2-regulated protein that promotes CD8 T cell dysfunction. Silencing PTGIR expression restores the anti-tumor function of KEAP1-deficient T cells. Moreover, lowering PTGIR expression in CD8 T cells both reduces terminal exhaustion and enhances T cell effector responses (i.e. IFN-gamma and granzyme production) to chronic infection and cancer. Together, these results establish the KEAP1-NRF2 axis as a metabolic sensor linking oxidative stress to CD8 T cell dysfunction and identify the prostacyclin receptor PTGIR as an NRF2-regulated immune checkpoint that regulates CD8 T cell fate decisions between effector and exhausted states.


