Role of Pseudomonas aeruginosa Dnr-regulated denitrification in oxic conditions
Role of Pseudomonas aeruginosa Dnr-regulated denitrification in oxic conditions
Balsam, S. S.; Mould, D. L.; Jean-Pierre, F.; Hogan, D. A.
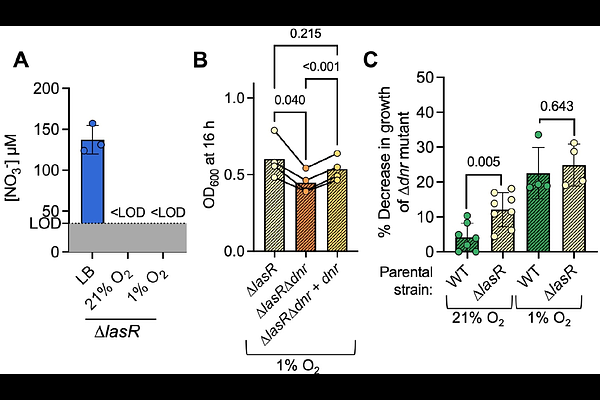
AbstractPseudomonas aeruginosa causes acute and chronic infections such as those that occur in the lungs of people with cystic fibrosis (CF). In infection environments, oxygen (O2) concentrations are often low. The transcription factor Anr responds to low O2 by upregulating genes necessary for P. aeruginosa fitness in microoxic and anoxic conditions. Anr regulates dnr, a gene encoding a transcriptional regulator that promotes the expression of genes required for using nitrate as an alternative electron acceptor during denitrification. In CF sputum, transcripts involved in denitrification are highly expressed. While Dnr is necessary for the anoxic growth of P. aeruginosa in CF sputum and artificial sputum media (ASMi), the contribution of denitrification to P. aeruginosa fitness in oxic conditions has not been well described. Here we show that P. aeruginosa requires dnr for fitness in ASMi and the requirement for dnr is abolished when nitrate is excluded from the media. Additionally, we show that P. aeruginosa consumes nitrate in lysogeny broth (LB) under microoxic conditions. Furthermore, strains without a functioning quorum sensing regulator LasR, which leads to elevated Anr activity, consume nitrate in LB even in normoxia. There was no growth advantage for P. aeruginosa when nitrate was present from 100 micromolar to low milimolar concentrations. However, P. aeruginosa consumption of nitrate in oxic conditions created a requirement for Dnr and Dnr-regulated NorCB likely due to the need to detoxify nitric oxide. These studies suggest that Anr- and Dnr-regulated processes may impact P. aeruginosa physiology in many common culture conditions.