Enhancer-driven local 3D chromatin domain folding modulates transcription in human mammary tumor cells
Enhancer-driven local 3D chromatin domain folding modulates transcription in human mammary tumor cells
Kocanova, S.; Raynal, F.; Goiffon, I.; Oksuz, B. A.; Bau, D.; Kamgoue, A.; Cantaloube, S.; Zhan, Y.; Lajoie, B.; Marti-Renom, M. A.; Dekker, J.; Bystricky, K.
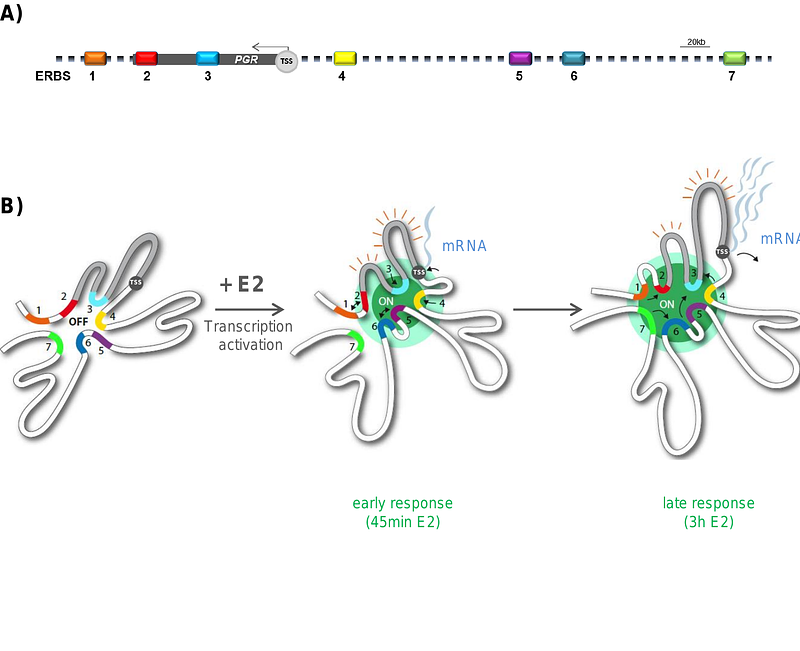
AbstractThe genome is organized in functional compartments and structural domains at the submegabase scale. How within these domains interactions between numerous cis-acting enhancers and promoters regulate transcription remains an open question. Here, we determined chromatin folding and composition over several hundred kb around estrogen responsive genes in human breast cancer cell lines following hormone stimulation. Modeling of 5C data at 1.8 kb resolution was combined with quantitative 3D analysis of multicolor FISH measurements at 100 nm resolution and integrated with ChIP-seq data on transcription factor binding and histone modifications. We found that rapid estradiol induction of the progesterone gene (PGR) expression occurs in the context of pre-existing, cell type specific chromosomal architectures encompassing the 90 kb PGR coding region and an enhancer-spiked 5' 300 kb upstream genomic region. In response to estradiol, interactions between estrogen-receptor alpha (ERa) bound regulatory elements are re-enforced. While initial enhancer-gene contacts coincide with RNA Pol 2 binding and transcription initiation, sustained hormone stimulation promotes ER accumulation creating a regulatory hub stimulating transcript synthesis. In addition to implications for estrogen receptor signaling, we uncover that preestablished chromatin architectures efficiently regulate gene expression upon stimulation without the need for de-novo extensive rewiring of long- range chromatin interactions.