A Comprehensive Workflow for Imaging Live Insulin Secretion Events and Granules in Intact Islets
A Comprehensive Workflow for Imaging Live Insulin Secretion Events and Granules in Intact Islets
Fye, M. A.; Sharma, R.; Myat, P. S. M.; Regan, P.; McKinney, H.; Gu, G.; Kaverina, I.
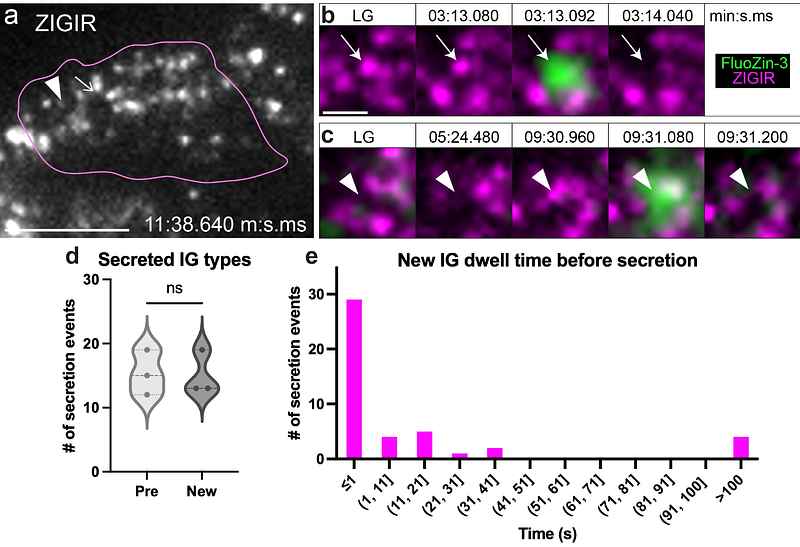
AbstractAccurate detection of insulin secretion from pancreatic beta cells is crucial for understanding normal physiological insulin secretion and its pathophysiological counterpart in diabetic states. Traditional methods using fluorescently labeled insulin granules or dye labeling often struggle to distinguish secretion from insulin granule dynamics. We present an optimized protocol using the cell-impermeable Zn2+-binding dye FluoZin-3, which fluoresces upon Zn2+ co-secretion with insulin outside of the islet, more accurately representing secretion. FluoZin-3 combined with intact islet attachment to vascular extracellular matrix and TIRF microscopy offers high spatial and temporal resolution as well as a high signal-to-noise ratio in a minimally perturbed system. Additionally, by integrating the cell-permeable Zn2+-binding dye ZIGIR, we can track insulin granule dynamics alongside secretion events. Our approach generates large datasets, which we efficiently analyze using ilastik machine learning software, enabling fast, accurate, and optionally supervised analysis. This technique builds on our previous protocols, detailing a streamlined workflow adaptable to high-resolution, live-cell microscopy for not just insulin but other secretory/granule systems as well. With this method, we investigated secretion behavior of different IG pools in real time during the first phase of insulin secretion: predocked, which appear before high glucose stimulation and are docked at the membrane; docked, which appear upon high glucose stimulation and dock at the membrane; and newcomer, which appear upon high glucose stimulation but do not dock at the membrane. The predocked and newcomer insulin granules are equally secreted and newcomer insulin granules dwell less than one second before secretion upon high glucose stimulation. The predocked and docked insulin granules, however, stay longer at the membrane before secretion. This method is useful for the investigation of functional beta cell heterogeneity of insulin granule secretion in space and time.